Rubber
Category : JEE Main & Advanced
It is a polymer which is capable of returning to its original length, shape or size after being stretched or deformed. It is the example of elastomer. Rubber are of two types.
(1) Natural rubber
(2) Synthetic rubber
(1) Natural rubber : It is obtained as latex from rubber trees. The latex is coagulated with acetic acid or formic acid. The coagulated mass is then squeezed.
The raw natural rubber is a soft gummy and sticky mass. It is insoluble in water, dil. Acids and alkalies but soluble in benzene, chloroform, ether, petrol and carbon disulphide. It absorb a large amount of water. It has low elasticity and tensile strength.
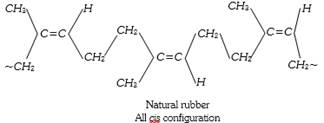
Destructive distillation of natural rubber gives mainly isoprene (2-methyl butadiene).
Thus isoprene is a monomer of natural rubber the no. of isoprene unit are 11,000 to 20,000 which linked together in a chain.
\[\underset{\text{Isopreme}}{\mathop{nC{{H}_{2}}=\overset{C{{H}_{3}}\,\,\,\,\,}{\mathop{\overset{|}{\mathop{C}}\,-CH}}\,}}\,=C{{H}_{2}}\xrightarrow{\text{Polymerisation}}\]\[\underset{\text{Natural rubber}}{\mathop{{{\left[ -C{{H}_{2}}-\overset{C{{H}_{3}}\,\,\,\,\,\,\,}{\mathop{\overset{|}{\mathop{C}}\,=CH}}\,-C{{H}_{2}}- \right]}_{n}}}}\,\]
(2) Synthetic rubber : The synthetic rubber is obtained by polymerising certain organic compounds which may have properties similar to rubber and some desirable properties. Most of these are derived from butadiene derivatives and contain carbon-carbon double bonds. The synthetic rubbers are either homopolymers of 1, 3 butadiene or copolymer in which one of the monomers is 1, 3 butadiene or its derivative so that the polymer has the availability of double bonds for its vulcanization. Some important examples are Neoprene, styrene, butadiene rubber (SBR) thiokol, silicones, polyurethane, rubber etc.
Vulcanization of rubber : The process of heating natural rubber with sulphur to improve its properties is called vulcanization. Vulcanization was introduced by Charles Goodyear.
Although natural rubber is thermoplastic substance in which there are no cross link between the polymer chain and it on vulcanization set into a given shape which is retained.
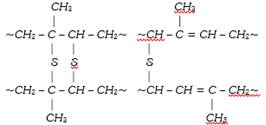
The vulcanization process performed originally was slow. Now a days, some additives such as zinc oxide etc. are used to accelerate the rate of vulcanization. During vulcanization, sulphur cross links are formed (figure) the double bonds in the rubber molecule acts as reactive sites. The allylic\[-C{{H}_{2}}\], alpha to double bond is also very reactive. During vulcanization, sulphur forms cross links at these reactive sites. As a result, rubber gets stiffened and intermolecular movement of rubber springs is prevented resulting in physical character of rubber. The extent of stiffness of vulcanized rubber depend upon the amount of sulphur added. For example about 5% sulphur is used for making tyre rubber while 30% of the sulphur is used for making battery case rubber.
In a polymer, the chains are normally tangled up with each other. When the rubber is stretched, the chains straighten out to some extent. The chains cannot slip past each other because of the polysulphide bridges. Thus, rubber can be stretched only to a limited extent. When the tension is removed, the chains try to coil up again and the rubber resumes its original shape.
The comparison of the main properties of natural rubber and vulcanized rubber are given below in the table,
| Natural rubber | Vulcanized rubber |
| (1) Natural rubber is soft and sticky | Vulcanized rubber is hard and non-sticky. |
| (2) It has low tensile strength. | It has high tensile strength. |
| (3) It has low elasticity. | It has high elasticity. |
| (4) It can be used over a narrow range of temperature (from \[{{10}^{o}}\] to \[{{60}^{o}}C\]). | It can be used over a wide range of temperature (\[-{{40}^{o}}\] to \[{{100}^{o}}C\]). |
| (5) It has low wear and tear resistance. | It has high wear and tear resistance. |
| (6) It is soluble in solvents like ether, carbon, tetrachloride, petrol, etc. | It is insoluble in all the common solvents. |
You need to login to perform this action.
You will be redirected in
3 sec
