Amines
Category : JEE Main & Advanced
Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group.
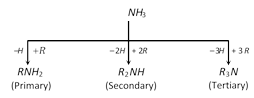
Amines are classified as primary, secondary or tertiary depending on the number of alkyl groups attached to nitrogen atom.
The characteristic groups in primary, secondary and tertiary amines are: \[\underset{\text{(amino)}}{\mathop{N{{H}_{2}}}}\,\]; \[\underset{\text{(imino)}}{\overset{|}{\mathop{NH}}}\,\]; \[\underset{(tert-\text{nitrogen)}}{\mathop{\underset{|}{\overset{|}{\mathop{-N\,\,\,}}}\,}}\,\]
In addition to above amines, tetra-alkyl derivatives similar to ammonium salts also exist which are called quaternary ammonium compounds.
\[N{{H}_{4}}I\]; \[\underset{\begin{smallmatrix} \,\,\,\,\,\,\text{Quaternary} \\ \text{ammonium }\,\text{iodide} \end{smallmatrix}}{\mathop{{{R}_{4}}NI}}\,\]; \[\underset{\begin{smallmatrix} \,\,\,\,\text{Tetramethyl} \\ \text{ammonium iodide} \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{4}}NI}}\,\] or \[\underset{\begin{smallmatrix} \,\,\,\,\text{Tetra-alkyl} \\ \text{ammonium salt} \end{smallmatrix}}{\mathop{{{\left[ R-\underset{\underset{R}{\mathop{|}}\,}{\overset{\overset{R}{\mathop{|}}\,}{\mathop{N}}}\,-R \right]}^{+}}}}\,{{X}^{-}}\]
(1) Simple and mixed amines : Secondary and tertiary amines may be classified as simple or mixed amines according as all the alkyl or aryl groups attached to the nitrogen atom are same or different. For example,
Simple amines : \[\underset{\text{Dimethylamine}}{\mathop{{{(C{{H}_{3}})}_{2}}NH}}\,\]; \[\underset{\text{Triethylamine}}{\mathop{{{(C{{H}_{3}}C{{H}_{2}})}_{3}}N}}\,\]
Mixed amines : \[\underset{\text{Ethylmethylamine}}{\mathop{{{C}_{2}}{{H}_{5}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{N}}\,H}}\,}}\,\]; \[\underset{\text{Methylaniline}}{\mathop{{{C}_{6}}{{H}_{5}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{N}}\,H}}\,}}\,\]
The aliphatic amines have pyramidal shape with one electron pair. In amines, N undergoes hybridisation.
(2) General methods of preparation
(i) Methods yielding mixture of amines (Primary, secondary and tertiary)
(a) Hofmann's method :The mixture of amines (\[{{1}^{o}},\,\,{{2}^{o}}\] and \[{{3}^{o}}\]) is formed by the alkylation of ammonia with alkyl halides.
\[\underset{\text{Methyliodi}\text{de}}{\mathop{C{{H}_{3}}I}}\,+N{{H}_{3}}\to \underset{\begin{smallmatrix} \text{Methylamine} \\ \,\,\,\,\,\text{(1}{}^\circ \text{)} \end{smallmatrix}}{\mathop{C{{H}_{3}}N{{H}_{2}}}}\,\xrightarrow{C{{H}_{3}}I}(\underset{\begin{smallmatrix} \text{Dimethylamine} \\ \,\,\,\,\,\,\,\text{(2}{}^\circ \text{)} \end{smallmatrix}}{\mathop{C{{H}_{3}}{{)}_{2}}NH}}\,\]
\[\xrightarrow{C{{H}_{3}}I}\underset{\begin{smallmatrix} \text{Trimethylamine} \\ \,\,\,\,\,\,\,\,\,\,\,\text{(3}{}^\circ \text{)} \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{3}}N}}\,\xrightarrow{C{{H}_{3}}I}\underset{\begin{smallmatrix} \,\,\,\,\text{Tetramethyl} \\ \text{ammonium iodide} \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{4}}NI}}\,\]
The primary amine may be obtained in a good yield by using a large excess of ammonia. The process is also termed as ammonolysis of alkyl halides. It is a nucleophilic substitution reaction.
(b) Ammonolysis of alcohols :
\[C{{H}_{3}}OH+N{{H}_{3}}\underset{350{}^\circ C}{\mathop{\xrightarrow{A{{l}_{2}}{{O}_{3}}}}}\,C{{H}_{3}}N{{H}_{2}}\]\[\xrightarrow{C{{H}_{3}}OH}{{(C{{H}_{3}})}_{2}}NH\xrightarrow{C{{H}_{3}}OH}{{(C{{H}_{3}})}_{3}}N\]
Primary amine may be obtained in a good yield by using a excess of ammonia.
(ii) Methods yielding primary amines
(a) Reduction of nitro compounds
\[R-N{{O}_{2}}+6[H]\underset{{Zn}/{HCl\text{ or }Ni\text{ or }LiAl{{H}_{4}}}\;}{\mathop{\xrightarrow{\,\,\,\,\,\,\,{Sn}/{HCl\text{ or}}\;\,\,\,\,\,\,}}}\,RN{{H}_{2}}+2{{H}_{2}}O\]
\[{{C}_{2}}{{H}_{5}}-N{{O}_{2}}+6[H]\to {{C}_{2}}{{H}_{5}}N{{H}_{2}}+2{{H}_{2}}O\]
(b) Reduction of nitriles (Mendius reaction)
\[R-C\equiv N+4[H]\to R-C{{H}_{2}}N{{H}_{2}}\]
\[\underset{\text{Methyl cyanide}}{\mathop{C{{H}_{3}}C\equiv N}}\,+4[H]\to \underset{\text{Ethylamine}}{\mathop{C{{H}_{3}}-C{{H}_{2}}N{{H}_{2}}}}\,\]
The start can be made from alcohol or alkyl halide.
\[\underset{\text{Alcohol}}{\mathop{R-OH}}\,\xrightarrow{SOC{{l}_{2}}}\underset{\text{Alkyl chloride}}{\mathop{R-Cl}}\,\xrightarrow{KCN}\underset{\text{Alkyl nitrile}}{\mathop{R-CN}}\,\underset{Na+{{C}_{2}}{{H}_{5}}OH}{\mathop{\xrightarrow{LiAl{{H}_{4}}or}}}\,\underset{\text{Primary amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\]\[\underset{\text{Alkyl nitrile}}{\mathop{R-CN}}\,\underset{Na+{{C}_{2}}{{H}_{5}}OH}{\mathop{\xrightarrow{LiAl{{H}_{4}}or}}}\,\underset{\text{Primary amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\]
This sequence gives an amine containing one more carbon atom than alcohol.
(c) By reduction of amides with \[LiAl{{H}_{4}}\]
\[RCON{{H}_{2}}\xrightarrow{LiAl{{H}_{4}}}RC{{H}_{2}}N{{H}_{2}}\]
\[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,\xrightarrow{LiAl{{H}_{4}}}\underset{\text{Ethylamine}}{\mathop{C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}}}\,\]
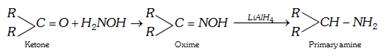
(d) By reduction of oximes : The start can be made from an aldehyde or ketone.
\[\underset{\text{Aldehyde}}{\mathop{RCHO}}\,\xrightarrow{{{H}_{2}}NOH}\underset{\text{Oxime}}{\mathop{RCH=NOH}}\,\underset{{or{{H}_{2}}}/{Ni}\;}{\mathop{\xrightarrow{LiAl{{H}_{4}}}}}\,\underset{\text{Primary amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\]
(e) Hofmann's bromamide reaction or degradation (Laboratory method) : By this method the amide \[(CON{{H}_{2}})\] group is converted into primary amino \[(\text{ }N{{H}_{2}})\] group.
\[R-\underset{\text{Amide}}{\mathop{CO-N{{H}_{2}}}}\,+B{{r}_{2}}+4KOH\to \underset{\text{Pri-amine}}{\mathop{R-N{{H}_{2}}}}\,+2KBr+{{K}_{2}}C{{O}_{3}}+2{{H}_{2}}O\]
This is the most convenient method for preparing primary amines.
This method gives an amine containing one carbon atom less than amide.
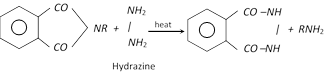
(f) Gabriel phthalimide synthesis : This method involves the following three steps.

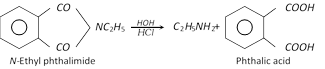
When hydrolysis is difficult, the N-alkyl phthalimide can be treated with hydrazine to give the required amine.
(g) By decarboxylation of a-amino acids
\[\underset{N{{H}_{2}}}{\mathop{R\underset{|}{\mathop{C}}\,HC}}\,OOH\underset{\text{heat}}{\mathop{\xrightarrow{Ba{{(OH)}_{2}}}}}\,RC{{H}_{2}}N{{H}_{2}}\]
\[\underset{\begin{smallmatrix} \alpha \text{-amino acetic acid} \\ \text{ (Glycine)} \end{smallmatrix}}{\mathop{\underset{N{{H}_{2}}}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,-COOH}}\,\underset{\text{heat}}{\mathop{\xrightarrow{Ba{{(OH)}_{2}}}}}\,\underset{\text{Methyl amine}}{\mathop{C{{H}_{3}}N{{H}_{2}}}}\,\]
(h) By means of a Grignard reagent and chloramine :
\[RMgX+ClN{{H}_{2}}\to RN{{H}_{2}}+MgXCl\]
(i) By hydrolysis of Isocyanides or Isocyanates
\[\underset{\text{Alkyl isocyanide}}{\mathop{R-\underset{H}{\overset{H}{\mathop{N}}}\,\equiv \underset{OH}{\overset{OH}{\mathop{C}}}\,}}\,+2{{H}_{2}}O\xrightarrow{(HCl)}\underset{\text{Alkyl amine}}{\mathop{R-N{{H}_{2}}}}\,+HCOOH\]
\[\underset{\text{methyl isonitile}}{\mathop{C{{H}_{3}}-NC}}\,+2HOH\xrightarrow{{{H}^{+}}}C{{H}_{3}}-N{{H}_{2}}+HCOOH\]
\[\underset{\text{Methyl isocyanate}}{\mathop{C{{H}_{3}}-\underset{H}{\overset{H}{\mathop{N}}}\,=\underset{OH}{\overset{OH}{\mathop{C}}}\,}}\,=O+2KOH\to C{{H}_{3}}-N{{H}_{2}}+{{K}_{2}}C{{O}_{3}}\]
\[\underset{\text{Alkyl isocyanate}}{\mathop{R-NCO}}\,+2KOH\to R-N{{H}_{2}}+{{K}_{2}}C{{O}_{3}}\]
(j) By Schmidt reaction :
\[\underset{\text{Acid}}{\mathop{R-COOH}}\,+\underset{\begin{smallmatrix} \text{Hydrazoic} \\ \,\,\,\,\text{acid} \end{smallmatrix}} {\mathop{{{N}_{3}}H}}\,\xrightarrow{Conc.{{H}_{2}}S{{O}_{4}}}\underset{\begin{smallmatrix} \text{Alkyl} \\ \text{amine} \end{smallmatrix}}{\mathop{R-N{{H}_{2}}}}\,+{{N}_{2}}+C{{O}_{2}}\]
In this reaction the acyl azide \[(RCO{{N}_{3}})\] and alkyl isocyanate \[(RNCO)\] are formed as an intermediate.
\[RCOOH+{{N}_{3}}H\to \underset{\text{Acyl azide}}{\mathop{RCO{{N}_{3}}}}\,+{{H}_{2}}O\]
\[\underset{\text{Acyl azide}}{\mathop{RCO{{N}_{3}}}}\,\to \underset{\text{Alkyl isocyanate}}{\mathop{R-N=C=O}}\,+{{N}_{2}}\]
\[R-N=C=O+{{H}_{2}}O\to \underset{\text{Alkyl amine}}{\mathop{R-N{{H}_{2}}}}\,+C{{O}_{2}}\]
The overall reaction which proceeds by the elimination of nitrogen from acyl azide followed by acidic or alkaline hydrolysis to yield primary amine containing one carbonless, is called Curtius Degradation.
The method uses acid chloride to prepare primary amine through acyl azide.
\[R\overset{\,O}{\mathop{-\overset{|\,|}{\mathop{C}}\,-}}\,OH\xrightarrow{SOC{{l}_{2}}}\underset{\text{Acyl chloride}}{\mathop{R\overset{\,O}{\mathop{-\overset{|\,|}{\mathop{C}}\,-}}\,Cl}}\,\xrightarrow{Na{{N}_{3}}}\underset{\text{Acyl azide}}{\mathop{R\overset{\,O}{\mathop{-\overset{|\,|}{\mathop{C}}\,-}}\,{{N}_{3}}}}\,\]
\[R\overset{\,O}{\mathop{-\overset{|\,|}{\mathop{C}}\,-}}\,{{N}_{3}}\xrightarrow{-{{N}_{2}}}R-N=C=O\underset{heat}{\mathop{\xrightarrow{2NaOH}}}\,R-N{{H}_{2}}+N{{a}_{2}}C{{O}_{3}}\]
The mechanism of curtius rearrangement is very similar to Hofmann degradation.
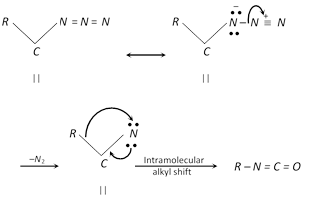
Schmidt reaction converts \[RCOOH\] to \[RN{{H}_{2}}\], which is a modification of curtius degradation. In this reaction a carboxylic acid is warmed with sodium azide \[(N{{a}^{+}}{{N}_{3}}^{})\] and conc. \[{{H}_{2}}S{{O}_{4}}\]. The carboxylic acid is directly converted to the primary amine without the necessity of isolating alkyl azide.
\[R\overset{\,O}{\mathop{-\overset{|\,|}{\mathop{C}}\,-}}\,OH\underset{heat}{\mathop{\xrightarrow{Na{{N}_{3}}+{{H}_{2}}S{{O}_{4}}(conc.)}}}\,RN{{H}_{2}}+{{N}_{2}}+C{{O}_{2}}\]
\[(Na{{N}_{3}}+{{H}_{2}}S{{O}_{4}}\to {{N}_{3}}H+NaHS{{O}_{4}})\]
(k) By Ritter reaction : It is a good method for preparing primary amines having a-tertiary alkyl group.
\[\underset{\text{Tert-butyl alcohol}}{\mathop{{{(C{{H}_{3}})}_{3}}C-OH}}\,+{{H}_{2}}S{{O}_{4}}+HCN\to \underset{\begin{smallmatrix} \text{Tert}-\text{butylamine} \\ \,\,\,\,\,\,\text{(1}{}^\circ \text{amine)} \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{3}}C-N{{H}_{2}}}}\,\]
\[\left[ {{R}_{3}}C-OH\xrightarrow{{{H}^{+}}}{{H}_{2}}O+\underset{\text{Tert-carboniumion}}{\mathop{{{R}_{3}}{{C}^{+}}}}\,\xrightarrow{HCN}{{R}_{3}}C\overset{+}{\mathop{N}}\, \right.\equiv CH\]\[\xrightarrow{{{H}_{2}}O}\left. CHO-{{R}_{3}}CNH\xrightarrow{O{{H}^{-}}}\underset{\text{Pri-amine}}{\mathop{{{R}_{3}}C-N{{H}_{2}}}}\,+HCO{{O}^{-}} \right]\]
(l) Reductive amination of aldehydes and ketones :
\[\underset{\text{Aldehyde}}{\mathop{R\overset{\,O}{\mathop{-\overset{|\,|}{\mathop{C}}\,-}}\,H}}\,+N{{H}_{3}}+{{H}_{2}}\underset{300atm}{\mathop{\xrightarrow{Ni,150{}^\circ C}}}\,\underset{\text{Primary amine}}{\mathop{R-C{{H}_{2}}-N{{H}_{2}}}}\,+{{H}_{2}}O\]
\[\left[ R\overset{H}{\mathop{-\overset{|}{\mathop{C}}\,=}}\,O+{{H}_{2}}HN\xrightarrow{(-{{H}_{2}}O)}[\underset{\text{Imine}}{\mathop{R\overset{H}{\mathop{-\overset{|}{\mathop{C}}\,=}}\,NH}}\,] \right.\]\[\left. \underset{Ni}{\mathop{\xrightarrow{{{H}_{2}}}}}\,RC{{H}_{2}}-N{{H}_{2}} \right]\]
\[\underset{\text{Ketone}}{\mathop{R\overset{\,O}{\mathop{-\overset{|\,|}{\mathop{C}}\,-}}\,C{{H}_{3}}}}\,+N{{H}_{3}}+{{H}_{2}}\underset{300atm}{\mathop{\xrightarrow{Ni,150{}^\circ C}}}\,R\overset{\,\,\,\,C{{H}_{3}}\,}{\mathop{-\overset{|}{\mathop{C}}\,H-}}\,N{{H}_{2}}\]
This reaction probably takes place through the formation of an imine (Schiff's base).
The primary amine can also be converted into sec. or tert. amines by the following steps
\[R-CHO+{R}'N{{H}_{2}}\xrightarrow{{{{H}_{2}}}/{Ni}\;}\underset{\text{Sec}\text{. amine}}{\mathop{RC{{H}_{2}}NH{R}'}}\,\]
\[RN{{H}_{2}}+2{{H}_{2}}C=O+2HCOOH\to \underset{\text{Tert}\text{.-amine}}{\mathop{RN{{(C{{H}_{3}})}_{2}}}}\,+2{{H}_{2}}O+2C{{O}_{2}}\]\[\to \underset{\text{Tert}\text{.-amine}}{\mathop{RN{{(C{{H}_{3}})}_{2}}}}\,+2{{H}_{2}}O+2C{{O}_{2}}\]
(m) By reduction of azide with \[NaB{{H}_{4}}\]
\[\underset{\begin{smallmatrix} \text{Alkyl halide} \\ \text{(1}{}^\circ \text{or2}{}^\circ \text{)} \end{smallmatrix}}{\mathop{R-X}}\,+\underset{\begin{smallmatrix} \text{Sodium} \\ \text{azide} \end{smallmatrix}}{\mathop{Na{{N}_{3}}}}\,\to \underset{\begin{smallmatrix} \text{Alkyl} \\ \text{azide} \end{smallmatrix}}{\mathop{R{{N}_{3}}}}\,\underset{{{H}_{2}}O}{\mathop{\xrightarrow{NaB{{H}_{4}}}}}\,\underset{\text{1}{}^\circ \text{amine}}{\mathop{RN{{H}_{2}}}}\,\]
(n) By Leuckart reaction : Aldehydes or ketones react with ammonium formate or with formamide to give formyl derivative of primary amine.
\[>C=O+\underset{\text{Amm}\text{.formate}}{\mathop{2HCOON{{H}_{4}}}}\,\to \,\,\,>CHNH\overset{\,O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,H\]\[+2{{H}_{2}}O+C{{O}_{2}}+N{{H}_{3}}\]
\[>C=O+\underset{\text{Formamide}}{\mathop{2HCON{{H}_{2}}}}\,\to \,\,\,\,>CHNH\overset{\,O}{\mathop{-\overset{|\,|}{\mathop{C}}\,-}}\,H+C{{O}_{2}}+N{{H}_{3}}\]
These formyl derivatives are readily hydrolysed by acid to yield primary amine.
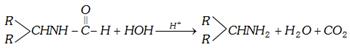
This is called Leuckart reaction, i.e.,
![]()
\[\underset{\text{Ethylene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,+N{{H}_{3}}\underset{450{}^\circ C,20atm}{\mathop{\xrightarrow{\text{Cobalt catalyst}}}}\,C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\]
(iii) Methods yielding secondary amines
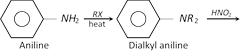
(a) Reaction of primary amines with alkyl halides
\[R-N{{H}_{2}}+R-X\xrightarrow{\Delta }{{R}_{2}}NH+HX\to \underset{\text{dialkyl ammonium salt}}{\mathop{{{R}_{2}}\overset{+}{\mathop{N}}\,{{H}_{2}}\overset{-}{\mathop{X}}\,}}\,\]
\[{{R}_{2}}\overset{+}{\mathop{N}}\,{{H}_{2}}\overset{-}{\mathop{X}}\,+NaOH\to \underset{\text{Secondary amine}}{\mathop{{{R}_{2}}NH}}\,+{{H}_{2}}O+NaX\]
(b) Reduction of isonitriles : \[\underset{\text{Alkyl isonitrile}}{\mathop{R-NC}}\,+4[H]\xrightarrow{Pt}\underset{\text{Sec}\text{. amine}}{\mathop{RNHC{{H}_{3}}}}\,\]
Secondary amine formed by this method always possesses one \[-C{{H}_{3}}\] group linked directly to nitrogen.
(c) Reaction of p-nitroso-dialkyl aniline with strong alkali solution :
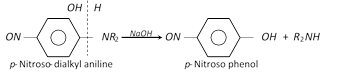
This is one of the best method for preparing pure secondary amines.
(d) Hydrolysis of dialkyl cyanamide
\[\left[ \underset{\begin{smallmatrix} \,\,\,\text{Calcium} \\ \text{cyanamide} \end{smallmatrix}}{\mathop{CaN-CN}}\,\xrightarrow{2NaOH}\underset{\begin{smallmatrix} \,\,\text{Sodium} \\ \text{cyanamide} \end{smallmatrix}}{\mathop{N{{a}_{2}}N-CN}}\,\xrightarrow{2RX}\underset{\begin{smallmatrix} \,\,\,\,\text{Dialkyl} \\ \text{cyanamide} \end{smallmatrix}}{\mathop{{{R}_{2}}N-CN}}\, \right]\]
\[{{R}_{2}}N-CN+2HOH\underset{O{{H}^{-}}}{\mathop{\xrightarrow{{{H}^{+}}or}}}\,\underset{\text{Dialkyl amine}}{\mathop{{{R}_{2}}NH}}\,+C{{O}_{2}}+N{{H}_{3}}\]
(e) Reduction of N-substituted amides : Reduction of N-substituted amides with \[LiAl{{H}_{4}}\] yields secondary amines.
Alkyl \[\beta -\]amino ketones are formed by the action of ketone with formaldehyde and \[N{{H}_{3}}\](or primary or secondary amines).
The product is referred to as Mannich base and the reaction is called Mannich Reaction.
\[C{{H}_{3}}COC{{H}_{3}}+HCHO+RN{{H}_{2}}\xrightarrow{heat}C{{H}_{3}}COC{{H}_{2}}C{{H}_{2}}NHR\]
Which can be reduced to alkyl amines.
\[\underset{N\text{-Alkyl acid amide}}{\mathop{R-CONH{R}'+}}\,4[H]\xrightarrow{LiAl{{H}_{4}}}\underset{\text{Sec}\text{.amine}}{\mathop{RC{{H}_{2}}NH{R}'}}\,+{{H}_{2}}O\]
(iv) Methods yielding tertiary amines
(a) Reaction of alkylhalides with ammonia
\[3RX+N{{H}_{3}}\to {{R}_{3}}N+3HX\to \underset{\text{Trialkyl ammonium salt}}{\mathop{{{R}_{3}}\overset{+}{\mathop{N}}\,H\overset{-}{\mathop{X}}\,}}\,\]
\[{{R}_{3}}\overset{+}{\mathop{N}}\,H\overset{-}{\mathop{X}}\,+NaOH\to {{R}_{3}}N+NaX+{{H}_{2}}O\]
(b) Reduction of N, N-disubstituted amides : The carbonyl group is converted into \[-C{{H}_{2}}\] group.
\[\underset{\begin{smallmatrix} N,N\text{-disubstituted} \\ \,\,\,\,\,\,\,\,\text{amide} \end{smallmatrix}}{\mathop{RCON{{{{R}'}}_{2}}}}\,\underset{4[H]}{\mathop{\xrightarrow{LiAl{{H}_{4}}}}}\,\underset{\text{ter}\text{. amine}}{\mathop{RC{{H}_{2}}N{{{{R}'}}_{2}}+}}\,{{H}_{2}}O\]
(c) Decomposition of tetra-ammonium hydroxides : The tetra-alkyl ammonium hydroxides are formed when corresponding halides are treated with moist silver oxide.
\[{{R}_{4}}\overset{+}{\mathop{N}}\,\bar{I}+AgOH\to {{R}_{4}}\overset{+}{\mathop{N}}\,O\overset{-}{\mathop{H}}\,+AgI\]
The hydroxides thus formed on heating decompose into tertiary amines. Tetramethyl ammonium hydroxide gives methyl alcohol as one of the products while all other tetra-alkyl ammonium hydroxides give an olefin and water besides tertiary amines.
\[{{(C{{H}_{3}})}_{4}}NOH\to {{(C{{H}_{3}})}_{3}}N+C{{H}_{3}}OH\]
\[{{(R)}_{4}}NOH\to {{(R)}_{3}}N+\text{olefin}+{{H}_{2}}O\]
(3) Separation of mixture of amines : When the mixture consists of salts of primary, secondary and tertiary amines along with quaternary salt, it is first distilled with KOH solution. The mixture of three amines distils over leaving behind non-volatile quaternary salt.
\[RN{{H}_{2}}.HI\,\text{or}\,RN{{\overset{+}{\mathop{H}}\,}_{3}}-\bar{I}+\overset{+}{\mathop{K}}\,O\overset{-}{\mathop{H}}\,\to \underset{\begin{smallmatrix} \text{Primary amine} \\ \text{(Volatile), Distillate} \end{smallmatrix}}{\mathop{RN{{H}_{2}}+KI}}\,\ +{{H}_{2}}O\]
\[{{R}_{2}}NH.HI\,\,\,\text{or}\,\,\,{{R}_{2}}N{{\overset{+}{\mathop{H}}\,}_{2}}-\bar{I}+\overset{+}{\mathop{K}}\,O\overset{-}{\mathop{H}}\,\to {{R}_{2}}NH+KI+{{H}_{2}}O\]
\[{{R}_{3}}N.HI\,\,\text{or}\,\,{{R}_{3}}N\overset{+}{\mathop{H}}\,-\bar{I}+\overset{+}{\mathop{K}}\,O\overset{-}{\mathop{H}}\,\to {{R}_{3}}N+KI+{{H}_{2}}O\]
\[{{R}_{4}}\overset{+}{\mathop{N}}\,\bar{I}\] (non-volatile tetra-alkyl ammonium salt) has no reaction with KOH, however remains as residue.
This mixture is separated into primary, secondary and tertiary amines by the application of following methods.
(i) Fractional distillation : The boiling points of primary, secondary and tertiary amines are quite different, i.e., the boiling point of \[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\] is \[17{}^\circ C,\text{ }{{({{C}_{2}}{{H}_{5}})}_{2}}\,NH\] is \[56{}^\circ C\] and \[{{({{C}_{2}}{{H}_{5}})}_{3}}N\] is \[95{}^\circ C\] and thus, these can be separated by fractional distillation. This method is used satisfactorily in industry.
(ii) Hofmann's method : The mixture of three amines is treated with diethyl oxalate. The primary amine forms a solid oxamide, a secondary amine gives a liquid oxamic ester while tertiary amine does not react.
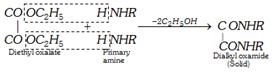
\[\underset{\text{Diethyl oxalate}}{\mathop{\underset{COO{{C}_{2}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{C}}\,OO{{C}_{2}}{{H}_{5}}}}\,}}\,+\underset{\begin{smallmatrix} \text{Secondary} \\ \,\,\text{amine} \end{smallmatrix}}{\mathop{HN{{R}_{2}}}}\,\xrightarrow{-{{C}_{2}}{{H}_{5}}OH}\underset{\begin{smallmatrix} \text{Dialkyl oxamic ester} \\ \text{ (liquid)} \end{smallmatrix}}{\mathop{\underset{COO{{C}_{2}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{C}}\,ON{{R}_{2}}\,\,\,\,}}\,}}\,\]
Primary amine is recovered when solid oxamide is heated with caustic potash solution and collected as distillate on distilling the reaction mixture.
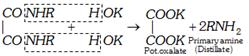
The liquid (mixture of oxamic ester+ tertiary amine) is subjected to fractional distillation when tertiary amine distils over.
The remaining liquid is distilled with KOH to recover secondary amine.
\[\underset{COO{{C}_{2}}{{H}_{5}}\ \ \ \ \ \ \ \ HOK}{\overset{CON{{R}_{2}}\ \ \ \ \ \ \ \ \ \ \ \ \ \ HOK}{\mathop{|\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ +\ \ \ \ \ \ \ \ \ }}}\,\to \underset{\begin{smallmatrix} \text{Secondary } \\ \,\,\,\text{amine} \end{smallmatrix}}{\mathop{{{R}_{2}}NH+}}\,\underset{\underset{\text{Pot}\text{. oxalate}}{\mathop{COOK}}\,}{\overset{COOK}{\mathop{|\,\,\,\,\,\,\,\,\,\,}}}\,+{{C}_{2}}{{H}_{5}}OH\]
(iii) Hinsberg's method : It involves the treatment of the mixture with benzene sulphonyl chloride, i.e., Hinsberg's reagent \[({{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl)\]. The solution is then made alkaline with aqueous alkali to form sodium or potassium salt of monoalkyl benzene sulphonamide (soluble in water).
\[{{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl+\underset{\begin{smallmatrix} \text{Primary} \\ \,\,\text{amine} \end{smallmatrix}}{\mathop{HNHR}}\,\to \underset{\begin{smallmatrix} N\text{-Alkyl benzene} \\ \,\text{sulphonamide} \end{smallmatrix}}{\mathop{{{C}_{6}}{{H}_{5}}S{{O}_{2}}NHR}}\,\]\[\xrightarrow{NaOH}\underset{\text{Soluble salt}}{\mathop{{{C}_{6}}{{H}_{5}}S{{O}_{2}}N(Na)R}}\,\]
The secondary amine forms N,N-dialkyl benzene sulphonamide which does not form any salt with NaOH and remains as insoluble in alkali solution.
\[{{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl\underset{\text{Sec}\text{. amine}}{\mathop{+HN{{R}_{2}}}}\,\to {{C}_{6}}{{H}_{5}}S{{O}_{2}}N{{R}_{2}}\]\[\xrightarrow{NaOH}\underset{\begin{smallmatrix} \text{(Insoluble in water,} \\ \text{soluble in ether)} \end{smallmatrix}}{\mathop{\text{No reaction }}}\,\]
Tertiary amine does not react.
The above alkaline mixture of the amines is extracted with ether.
Two distinct layers are formed. Lower layer, the aqueous layer consists of sodium salt of N-alkyl benzene sulphonamide (primary amine) and upper layer, the ether layer consists of N, N-dialkyl benzene sulphonamide (secondary amine) and tertiary amine.
Two layers are separated. The upper layer is fractionally distilled. One fraction obtained is tertiary amine and the other fraction is treated with concentrated HCl to recover secondary amine hydrochloride which gives free secondary amine on distillation with NaOH.
\[{{C}_{6}}{{H}_{5}}S{{O}_{2}}N{{R}_{2}}+HCl+{{H}_{2}}O\to {{C}_{6}}{{H}_{5}}S{{O}_{2}}.OH+{{R}_{2}}NH.HCl\]
\[{{R}_{2}}NH.HCl+NaOH\,\to \underset{\text{Sec}\text{. amine}}{\mathop{{{R}_{2}}NH+}}\,NaCl+{{H}_{2}}O\]
The aqueous layer is acidified and hydrolysed with dilute HCl. The hydrochloride formed is then distilled with NaOH when primary amine distils over.
\[{{C}_{6}}{{H}_{5}}S{{O}_{2}}N(Na)R+HCl\to \underset{\begin{smallmatrix} \text{Sulphonamide of} \\ \text{primary amine} \end{smallmatrix}}{\mathop{{{C}_{6}}{{H}_{5}}S{{O}_{2}}NHR+}}\,NaCl\] \[{{C}_{6}}{{H}_{5}}S{{O}_{2}}NHR+HCl+{{H}_{2}}O\to {{C}_{6}}{{H}_{5}}S{{O}_{2}}.OH\underset{\begin{smallmatrix} \text{Primary amine} \\ \text{hydrochloride} \end{smallmatrix}}{\mathop{+RN{{H}_{2}}.HCl}}\,\]
\[RN{{H}_{2}}.HCl+NaOH\to RN{{H}_{2}}+NaCl+{{H}_{2}}O\]
(4) Physical properties
(i) Lower amines are gases or low boiling point liquids and possess a characteristic ammonia like smell (fishy odour). Higher members are solids.
(ii) The boiling points rise gradually with increase of molecular mass. Amines are polar compounds like \[N{{H}_{3}}\] and have comparatively higher boiling points than non-polar compounds of similar molecular masses. This is due to the presence of intermolecular hydrogen bonding.
\[\underset{\text{Hydrogen bonding in amines}}{\mathop{H\underset{R}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{N}}}\,}}}\,:---H\underset{R}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{N}}}\,}}}\,:---H\underset{R}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{N}}}\,}}}\,:---}}\,\]
(iii) Amines are soluble in water. This is due to hydrogen bonding between amine and water molecules. Amines are also soluble in benzene and ether.
\[\underset{\text{Hydrogen bonding between amine and water molecules}}{\mathop{H\underset{\underset{H}{\mathop{|}}\,}{\mathop{{\ddot{O}}}}\,:---H\underset{R}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{N}}}\,}}}\,:---H\underset{\underset{H}{\mathop{|}}\,}{\mathop{{\ddot{O}}}}\,:---H\underset{R}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{N}}}\,}}}\,:---}}\,\]
Solubility decreases with increase of molecular mass.
(5) Chemical properties : The main reactions of amines are due to the presence of a lone pair of electrons on nitrogen atom. Amines are electrophilic reagents as the lone pair of electrons can be donated to electron seeking reagents, (i.e., electrophiles).
Except the amines containing tertiary butyl group, all lower aliphatic amines are stronger bases than ammonia because of + I (inductive) effect. The alkyl groups, which are electron releasing groups, increase the electron density around the nitrogen thereby increasing the availability of the lone pair of electrons to proton or Lewis acids and making the amine more basic (larger Kb). Thus, it is expected that the basic nature of amines should be in the order tertiary > secondary > primary, but the observed order in the case of lower members is found to be as secondary > primary > tertiary. This anomalous behaviour of tertiary amines is due to steric factors, i.e., crowding of alkyl groups cover nitrogen atom from all sides and thus makes the approach and bonding by a proton relatively difficult which results the maximum steric strain in tertiary amines. The electrons are there but the path is blocked, resulting the reduced in its basicity.
(i) The order of basic nature of various amines has been found to vary with nature of alkyl groups.
| Alkyl group | Relative strength |
| \[C{{H}_{3}}\] | \[{{R}_{2}}NH>RN{{H}_{2}}>{{R}_{3}}N>N{{H}_{3}}\] |
| \[{{C}_{2}}{{H}_{5}}\] | \[{{R}_{2}}NH>RN{{H}_{2}}>N{{H}_{3}}>{{R}_{3}}N\] |
| \[{{(C{{H}_{3}})}_{2}}CH\text{ }\] | \[RN{{H}_{2}}>N{{H}_{3}}>{{R}_{2}}NH>{{R}_{3}}N\] |
| \[{{(C{{H}_{3}})}_{3}}C\text{ }\] | \[N{{H}_{3}}>RN{{H}_{2}}>{{R}_{2}}NH>{{R}_{3}}N\] |
(ii) Basic nature of aromatic amines : In aniline or other aromatic amines, the lone pair present on nitrogen atom is delocalized with benzene ring by resonance.
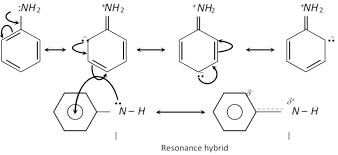
But anilinium ion is less resonance stabilized than aniline.

Thus, electron density is less on N atom due to which aniline or other aromatic amines are less basic than aliphatic amines.
However, any group which when present on benzene ring has electron withdrawing effect \[(\text{ }N{{O}_{2}},~CN,S{{O}_{3}}H,COOHCl,{{C}_{6}}{{H}_{5}},\,\,etc.)\] decreases basicity of aniline (Nitroaniline is less basic than aniline as nitro group is electron withdrawing group (– I group) and aniline is more basic than diphenyl amine), while a group which has electron repelling effect \[(N{{H}_{2}},OR,R,\,etc.)\] increases basicity of aniline. Toluidine is more basic than aniline as \[-C{{H}_{3}}\] group is electron repelling group (+ I group).
Further greater the value of \[{{K}_{b}}\] or lower the value of \[p{{K}_{b}},\] stronger will be the base. The basic character of some amines have the following order,
\[{{R}_{2}}NH>RN{{H}_{2}}>{{C}_{6}}{{H}_{5}}C{{H}_{2}}N{{H}_{2}}>N{{H}_{3}}>{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
N-alkylated anilines are stronger bases than aniline because of steric effect. Ethyl group being bigger than methyl has more steric effect, so N-ethyl aniline is stronger base than N-methyl aniline. Thus, basic character is,
\[{{C}_{6}}{{H}_{5}}N{{({{C}_{2}}{{H}_{5}})}_{2}}>{{C}_{6}}{{H}_{5}}NH{{C}_{2}}{{H}_{5}}>{{C}_{6}}{{H}_{5}}N{{(C{{H}_{3}})}_{2}}\]\[>{{C}_{6}}{{H}_{5}}NHC{{H}_{3}}>{{C}_{6}}{{H}_{5}}N{{H}_{2}}N{{H}_{3}}>{{C}_{6}}{{H}_{5}}NH{{C}_{2}}{{H}_{5}}\]\[>{{C}_{6}}{{H}_{5}}NHC{{H}_{3}}>{{C}_{6}}{{H}_{5}}N{{H}_{2}}>{{C}_{6}}{{H}_{5}}NH{{C}_{6}}{{H}_{5}}\]
In Toluidines –p-isomer \[a>m->o-\]
Chloroanilines–p-isomer \[>m->o-\]
Phenylene diamines –p-isomer \[>m->o-\]
Nitroanilines–m-isomer \[>p->o-\]
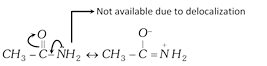
\[\underset{(s{{p}^{3}})}{\mathop{C{{H}_{3}}\ddot{N}{{H}_{2}}}}\,>C{{H}_{3}}\underset{(s{{p}^{2}})}{\mathop{\ddot{N}=CHC}}\,{{H}_{3}}>\underset{(sp)}{\mathop{C{{H}_{3}}C\equiv }}\,\ddot{N}\]\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}>{{H}_{2}}C\]\[=CHC{{H}_{2}}N{{H}_{2}}>HC\equiv CC{{H}_{2}}N{{H}_{2}}\] \[{{(C{{H}_{3}})}_{2}}NH>C{{H}_{3}}N{{H}_{2}}>N{{H}_{3}}>{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
\[{{(C{{H}_{3}})}_{2}}NH>C{{H}_{3}}N{{H}_{2}}>{{C}_{6}}{{H}_{5}}NHC{{H}_{3}}>{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
\[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}>HO{{(C{{H}_{2}})}_{3}}N{{H}_{2}}>HO{{(C{{H}_{2}})}_{2}}N{{H}_{2}}\]
\[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}>{{C}_{6}}{{H}_{5}}CON{{H}_{2}}>C{{H}_{3}}CON{{H}_{2}}\]
(iii) Salt formation : Amines being basic in nature, combine with mineral acids to form salts.
\[R-N{{H}_{2}}+HCl\to \underset{\begin{smallmatrix} \text{Alkylammon}\text{ium } \\ \text{ chloride} \end{smallmatrix}}{\mathop{R\overset{+}{\mathop{N}}\,{{H}_{3}}\bar{C}l}}\,\]
\[2RN{{H}_{2}}+{{H}_{2}}S{{O}_{4}}\to \underset{\text{Alkylammonium sulphate}}{\mathop{{{(R\overset{+}{\mathop{N}}\,{{H}_{3}})}_{2}}SO_{4}^{}}}\,\]
(iv) Nature of aqueous solution : Solutions of amines are alkaline in nature.
\[RN{{H}_{2}}+HOH\]? \[R\overset{+}{\mathop{N}}\,{{H}_{3}}O{{H}^{}}\]? \[{{[RN{{H}_{3}}]}^{+}}+O{{H}^{}}\]
\[{{R}_{2}}NH+HOH\]? \[{{R}_{2}}\overset{+}{\mathop{N}}\,{{H}_{2}}O{{H}^{}}\]? \[{{[{{R}_{2}}N{{H}_{2}}]}^{+}}+O{{H}^{}}\]
\[{{R}_{3}}N+HOH\] ? \[{{R}_{3}}\overset{+}{\mathop{N}}\,HO{{H}^{}}\]? \[{{[{{R}_{3}}NH]}^{+}}+O{{H}^{}}\]
The aqueous solutions of amines behaves like \[N{{H}_{4}}OH\] and give ferric hydroxide precipitate with ferric chloride and blue solution with copper sulphate.
\[3RN{{H}_{3}}OH+FeC{{l}_{3}}\to Fe{{(OH)}_{3}}+3RN{{H}_{3}}Cl\]
(v) Reaction with alkyl halides (Alkylation)
\[\underset{\text{Pri}\text{.amine}}{\mathop{RN{{H}_{2}}}}\,\underset{-HX}{\mathop{\xrightarrow{{R}'X}}}\,\underset{\text{Sec}\text{.a}\text{mine}}{\mathop{RNH{R}'}}\,\underset{-HX}{\mathop{\xrightarrow{{R}'X}}}\,\underset{\text{Tert}\text{. a}\text{mine}}{\mathop{RN{{{{R}'}}_{2}}}}\,\xrightarrow{{R}'X}\underset{\text{Q}\text{uaternary sa}\text{lt}}{\mathop{(R\overset{+}{\mathop{N}}\,{{{{R}'}}_{3}}){{X}^{}}}}\,\]
(vi) Reaction with acetyl chloride (Acylation)
\[\underset{\text{Pri}\text{. a}\text{mine}}{\mathop{RN{{H}_{2}}}}\,+ClOCC{{H}_{3}}\xrightarrow{HCl}\underset{N\text{-Alkyl acetamide}}{\mathop{RNHOCC{{H}_{3}}}}\,\]
\[\underset{\text{Sec}\text{. a}\text{mine}}{\mathop{{{R}_{2}}NH+}}\,ClOCC{{H}_{3}}\xrightarrow{HCl}\underset{N,N\text{-Dialkyl acetamide}}{\mathop{{{R}_{2}}NOCC{{H}_{3}}}}\,\]
Tertiary amines do not react since they do not have replaceable hydrogen on nitrogen.
Therefore, all these above reactions are used to distinguish between \[{{1}^{o}},\,{{2}^{o}}\] and \[{{3}^{o}}\]-amines.
(vii) Action of sodium
\[\underset{{{1}^{o}}\text{amine}}{\mathop{2RN{{H}_{2}}}}\,+2Na\xrightarrow{\Delta }\underset{\text{Sod}\text{. salt}}{\mathop{2{{[RNH]}^{}}}}\,N{{a}^{+}}+{{H}_{2}}\uparrow \]
\[\underset{{{\text{2}}^{o}}\text{amine}}{\mathop{2{{R}_{2}}NH}}\,+2Na\xrightarrow{\Delta }\underset{\text{Sod}\text{.sa}\text{lt}}{\mathop{2{{[{{R}_{2}}N]}^{}}N{{a}^{+}}}}\,+{{H}_{2}}\uparrow \]
(viii) Action of halogens
\[\underset{\text{Alkyl amine}}{\mathop{RN{{H}_{2}}}}\,\underset{NaOH}{\mathop{\xrightarrow{{{X}_{2}}}}}\,RNHX\underset{NaOH}{\mathop{\xrightarrow{{{X}_{2}}}}}\,\underset{\begin{smallmatrix} \text{Dihalo-alkyl} \\ \,\,\,\text{amine} \end{smallmatrix}}{\mathop{RN{{X}_{2}}}}\,\] \[\underset{\text{Dia}\text{lkyl amine}}{\mathop{{{R}_{2}}NH}}\,\underset{NaOH}{\mathop{\xrightarrow{{{X}_{2}}}}}\,\underset{\begin{smallmatrix} \text{Halo-d}\text{ialkyl} \\ \,\,\,\text{amine} \end{smallmatrix}}{\mathop{{{R}_{2}}NX}}\,\]
(ix) Reaction with Grignard reagent
![]()
\[{{R}_{2}}NH+C{{H}_{3}}MgI\to C{{H}_{4}}+{{R}_{2}}NMgI\]
(x) Carbylamine reaction : This reaction is shown by only primary amines. This is a test of primary amines and is used to distinguish primary amines from secondary and tertiary amines.
\[RN{{H}_{2}}+CHC{{l}_{3}}+\underset{\text{(Alc}\text{.)}}{\mathop{3KOH}}\,\to \underset{\begin{smallmatrix} \text{Alkyl isocyanide} \\ \text{ (carbyl amine)} \end{smallmatrix}}{\mathop{RNC}}\,+3KCl+3{{H}_{2}}O\]
Isocyanides are bad smelling compounds and can be easily detected.
(xi) Reaction with nitrous acid
(a) Primary amines form alcohols with nitrous acid \[(NaN{{O}_{2}}+\text{ }HCl)\]. Nitrogen is eliminated.
\[\underset{\text{Pri}\text{. amine}}{\mathop{RN{{H}_{2}}+}}\,HONO\to \underset{\text{Alcohol}}{\mathop{ROH}}\,+{{N}_{2}}+{{H}_{2}}O\]
Methyl amine is an exception to this reaction, i.e.,
\[C{{H}_{3}}N{{H}_{2}}+2HONO\to C{{H}_{3}}\underset{\text{Methyl nitrite}}{\mathop{ON=O+}}\,{{N}_{2}}+2{{H}_{2}}O\]
\[2C{{H}_{3}}N{{H}_{2}}+2HONO\to \underset{\text{Dimethyl ether}}{\mathop{C{{H}_{3}}OC{{H}_{3}}}}\,+2{{N}_{2}}+3{{H}_{2}}O\]
(b) Secondary amines form nitrosoamines which are water insoluble yellow oily liquids.
\[\underset{\text{Sec}\text{. amine}}{\mathop{{{R}_{2}}NH+}}\,HONO\to \underset{\begin{smallmatrix} \,\,\,\,\,\text{Dialkyl} \\ \text{nitrosoamine} \end{smallmatrix}}{\mathop{{{R}_{2}}NNO}}\,+{{H}_{2}}O\]
Nitrosoamine on warming with phenol and conc. \[{{H}_{2}}S{{O}_{4}}\] give a brown or red colour which soon changes to blue green. The colour changes to red on dilution and further changes to blue or violet with alkali. This colour change is referred to Liebermann's nitroso reaction and is used for the test of secondary amines.
(c) Tertiary amines react nitrous acid to form nitrite salts which are soluble in water. These salts on heating give alcohols and nitrosoamines.
\[\underset{\text{Tert}\text{.amine}}{\mathop{{{R}_{3}}N+}}\,HONO\to \underset{\text{Trialkyl ammoniumnitrite}}{\mathop{{{[{{R}_{3}}NH]}^{+}}NO_{2}^{}}}\,\xrightarrow{heat}\underset{\text{Alcohol}}{\mathop{ROH}}\,+\underset{\text{Nitrosoamine}}{\mathop{{{R}_{2}}NN=O}}\,\]
This reaction (nitrous acid test) is used to make distinction between primary, secondary and tertiary amines.
(xii) Reaction with carbon di sulphide : This Hofmann’s mustard oil reaction is used as a test for primary amines.
![]()
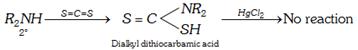
(xiii) Oxidation : All the three types of amines undergo oxidation. The product depends upon the nature of oxidising agent, class of amine and the nature of the alkyl group.
(a) Oxidation of primary amines
\[\underset{\text{Pri}\text{. amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\underset{KMn{{O}_{4}}}{\mathop{\xrightarrow{[O]}}}\,\underset{\text{Aldimine}}{\mathop{RCH=NH}}\,\xrightarrow{{{H}_{2}}O}\underset{\text{Aldehyde}}{\mathop{RCHO}}\,+N{{H}_{3}}\]
\[{{R}_{2}}CHN{{H}_{2}}\underset{KMn{{O}_{4}}}{\mathop{\xrightarrow{[O]}}}\,\underset{\text{Ketimine}}{\mathop{{{R}_{2}}C=NH}}\,\xrightarrow{{{H}_{2}}O}\underset{\text{Ketone}}{\mathop{{{R}_{2}}CO}}\,+N{{H}_{3}}\]
(b) Oxidation of secondary amines
\[\underset{\text{Sec}\text{. amine}}{\mathop{{{R}_{2}}NH}}\,\underset{KMn{{O}_{4}}}{\mathop{\xrightarrow{[O]}}}\,\underset{\text{Tetra-alkyl hydrazine}}{\mathop{{{R}_{2}}NN{{R}_{2}}}}\,\]; \[{{R}_{2}}NH\underset{{{H}_{2}}S{{O}_{5}}}{\mathop{\xrightarrow{[O]}}}\,\underset{\text{Dialkyl hydroxylamine}}{\mathop{{{R}_{2}}NOH}}\,\]
(c) Oxidation of tertiary amines : Tertiary amines are not oxidised by potassium permanganate but are oxidised by Caro's acid or Fenton's reagent to amine oxides.
\[\underset{\text{Tert}\text{. amine}}{\mathop{{{R}_{3}}N+[O]}}\,\to \underset{\text{Amine oxide}}{\mathop{[{{R}_{3}}N\to O]}}\,\]
(xiv) Reaction with other electrophilic reagents
\[\underset{\text{Pri}\text{. amine}}{\mathop{RN{{H}_{2}}+}}\,\underset{\text{Aldehyde}}{\mathop{O=CH{R}'}}\,\to \underset{\text{Schiff }\!\!'\!\!\text{ s base}}{\mathop{RN=CH}}\,{R}'\] \[2RN{{H}_{2}}+\underset{\begin{smallmatrix} \text{Carbonyl} \\ \,\,\text{chloride} \end{smallmatrix}}{\mathop{Cl\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,Cl}}\,\to \underset{\begin{smallmatrix} \,\,\,\text{Dialkyl urea} \\ \text{(Symmetrical)} \end{smallmatrix}}{\mathop{RNH\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,NHR}}\,+2HCl\] \[RNHH\ \underset{\text{Isocyanate}}{\mathop{+O=C=N{R}'}}\,\to \underset{\begin{smallmatrix} \,\,\,\,\text{Dialkyl urea} \\ \text{(Unsymmetrical)} \end{smallmatrix}}{\mathop{RNH\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,HN{R}'}}\,\]
\[RNHH+\underset{\text{Isothiocyanate}}{\mathop{S=C=N{R}'}}\,\to \underset{\text{Dialkyl thiourea}}{\mathop{RNH\overset{S}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,NH{R}'}}\,\]
(xv) Ring substitution in aromatic amines : Aniline is more reactive than benzene. The presence of amino group activates the aromatic ring and directs the incoming group preferably to ortho and para positions.
(a) Halogenation
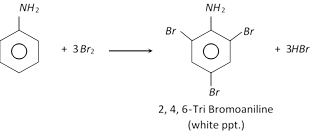
This reaction is used as a test for aniline.
However, if monosubstituted derivative is desired, aniline is first acetylated with acetic anhydride and then halogenation is carried out. After halogenation, the acetyl group is removed by hydrolysis and only monosubstituted halogen derivative is obtained.
It may be noted that \[-N{{H}_{2}}\] group directs the attacking group at \[o-\] and \[p-\] positions and therefore, both \[o-\] and \[p-\] derivatives are obtained.
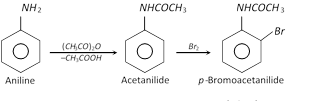
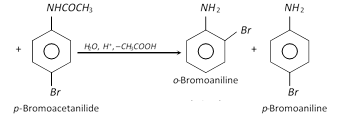
Acetylation deactivates the ring and controls the reaction to monosubstitution stage only because acetyl group is electron withdrawing group and therefore, the electron pair of N-atom is withdrawn towards the carbonyl group.
(b) Nitration : Aromatic amines cannot be nitrated directly because they are readily oxidized. This is because, \[HN{{O}_{3}}\] is a strong oxidising agent and results in partial oxidation of the ring to form a black mass.
Therefore, to solve this problem, nitration is carried out by protecting the \[-N{{H}_{2}}\] group by acetylation. The acetylation deactivates the ring and therefore, controls the reaction.
The hydrolysis of nitroacetanilides removes the protecting acyl group and gives back amines.

(c) Sulphonation
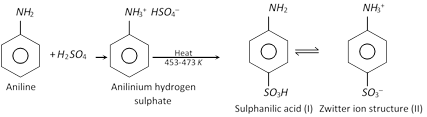
The sulphanilic acid exists as a dipolar ion (structure II) which has acidic and basic groups in the same molecule. Such ions are called Zwitter ions or inner salts.
(6) Uses
(i) Ethylamine is used in solvent extraction processes in petroleum refining and as a stabiliser for rubber latex.
(ii) The quaternary ammonium salts derived from long chain aliphatic tertiary amines are widely used as detergents.
(iii) Aliphatic amines of low molecular mass are used as solvents.
Distinction between primary, secondary and tertiary amines
| Test | Primary amine | Secondary amine | Tertiary amine |
| Action of \[CHC{{l}_{3}}\] and alcoholic KOH. (Carbylamine test) | Bad smelling carbylamine (Isocyanide) is formed. | No action. | No action. |
| Action of \[C{{S}_{2}}\] and \[HgC{{l}_{2}}\]. (Mustard oil test) | Alkyl isothiocyanate is formed which has pungent smell like mustard oil. | No action. | No action |
| Action of nitrous acid. | Alcohol is formed with evolution of nitrogen. | Forms nitrosoamine which gives green colour with phenol and conc. \[{{H}_{2}}S{{O}_{4}}\] (Liebermann's test). | Forms nitrite in cold which on heating gives nitrosoa- mine which responds to Liebermann's test. |
| Action of acetyl chloride. | Acetyl derivative is formed. | Acetyl derivative is formed. | No action. |
| Action of Hinsberg's reagent. | Monoalkyl sulphonamide is formed which is soluble in KOH. | Dialkyl sulphonamide is formed which is insoluble in KOH. | No action. |
| Action of methyl iodide. | 3 molecules (moles) of \[C{{H}_{3}}I\] to form quaternary salt with one mole of primary amine. | 2 moles of \[C{{H}_{3}}I\] to form quaternary salt with one mole of secondary amine. | One mole of \[C{{H}_{3}}I\] to form quaternary salt with one mole of tertiary amine. |
You need to login to perform this action.
You will be redirected in
3 sec
