Halo-Arenes
Category : JEE Main & Advanced
In these compounds the halogen is linked directly to the carbon of the benzene nucleus.
(1) Nomenclature : Common name is aryl halide IUPAC name is halo-arene.
Example :
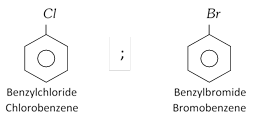
(2) Methods of preparation
(i) By direct halogination of benzene ring
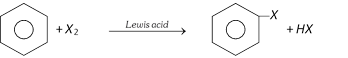
Lewis acid \[=Fe{{X}_{3}},Al{{X}_{3}},\,Tl{{(OAC)}_{3}}\]; \[{{X}_{2}}=C{{l}_{2}},B{{r}_{2}}\]
(ii) From diazonium salts
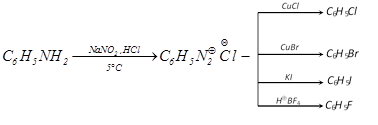
(iii) Hunsdiecker reaction :
\[{{C}_{6}}{{H}_{5}}CO{{O}^{-}}A{{g}^{+}}\xrightarrow{B{{r}_{2}}}{{C}_{6}}{{H}_{5}}Br+C{{O}_{2}}+AgBr\]
(iv) From Aryl thalium compound :
\[ArH+Tl{{(OOCC{{F}_{3}})}_{3}}\xrightarrow[-C{{F}_{3}}C{{O}_{2}}H]{}\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{Aryl thallium trifluoroacetate}}{\mathop{ArTl{{(OOC{{F}_{3}})}_{2}}\underset{\Delta }{\mathop{\xrightarrow{KI}}}\,ArI}}\,\] \[\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{Aryl thallium trifluoroacetate}}{\mathop{ArTl{{(OOC{{F}_{3}})}_{2}}\underset{\Delta }{\mathop{\xrightarrow{KI}}}\,ArI}}\,\]
(3) Physical properties
(i) Physical state : Haloarenes are colourless liquid or crystalline solid.
(ii) Solubility : They are insoluble in water, but dissolve readily in organic solvents. Insolubility is due to inability to form hydrogen bonding in water. Para isomer is less soluble than ortho isomer.
(iii) Halo-arenes are heavier than water.
(iv) B.P. of halo-arenes follow the trend. Iodo arene > Bromo arene > Chloro arene.
(4) Chemical properties
Inert nature of chlorobenzene : Aryl halides are unreactive as compared to alkyl halides as the halogen atom in these compounds is firmly attached and cannot be replaced by nucleophiles. Such as \[O{{H}^{-}},NH_{2}^{-},C{{N}^{-}}\] etc.
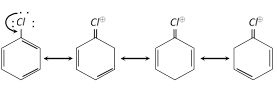
Thus delocalization of electrons by resonance in aryl halides, brings extra stability and double bond character between \[C-X\]bond. This makes the bond stronger and shorter than pure single bond. However under vigorous conditions the following nucleophilic substitution reactions are observed,
(i) Nucleophilic displacement :
\[{{C}_{6}}{{H}_{5}}Cl\underset{500\,atm.}{\mathop{\xrightarrow{NaOH,\,350{}^\circ C}}}\,{{C}_{6}}{{H}_{5}}OH+NaCl\]
(ii) Electrophilic aromatic substitution
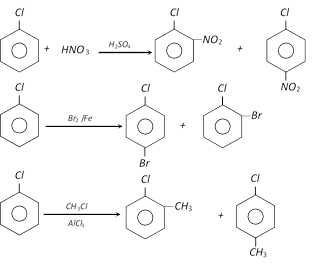
(iii) Wurtz – fittig reaction :
\[{{C}_{6}}{{H}_{5}}Br+C{{H}_{3}}Br\underset{\text{Ether}}{\mathop{\xrightarrow{Na}}}\,{{C}_{6}}{{H}_{5}}C{{H}_{3}}+2NaBr\]
(iv) Formation of grignard reagent :
\[{{C}_{6}}{{H}_{5}}Br\underset{\text{Ether}}{\mathop{\xrightarrow{Mg}}}\,{{C}_{6}}{{H}_{5}}MgBr\]
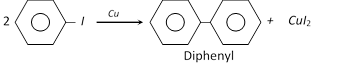
(v) Ullmann reaction
You need to login to perform this action.
You will be redirected in
3 sec
