Unsaturated Dicarboxylic Acids
Category : JEE Main & Advanced
The molecular formula of the simplest unsaturated dicarboxylic acid is \[HOOC.CH=CH.COOH\]. This formula, however represents two chemical compounds, maleic acid and fumaric acid, which are geometrical isomers.
\[\underset{Cis\text{-form (Maleic acid)}}{\mathop{\underset{\,H-C-COOH}{\mathop{H-\underset{|\,|}{\mathop{C}}\,-COOH}}\,}}\,\] \[\underset{Trans\text{-form (Fumaric acid)}}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\mathop{HOOC-\underset{|\,|}{\mathop{C}}\,-H}}\,}}\,\]
(1) Methods of Preparation of Maleic Acid
(i) By catalytic oxidation of 2-butene or benzene
\[\underset{2-\text{Butene}}{\mathop{\underset{CH-C{{H}_{3}}}{\overset{CH-C{{H}_{3}}}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,+{{30}_{2}}\underset{400{}^\circ C}{\mathop{\xrightarrow{{{V}_{2}}{{O}_{5}}}}}\,\underset{\text{Maleic acid}}{\mathop{\underset{CHCOOH}{\overset{CHCOOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,+2{{H}_{2}}O\]
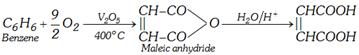
(ii) From malic acid :
\[\underset{\text{boil}}{\mathop{\xrightarrow{NaOH}}}\,\underset{\text{Sodium salt}}{\mathop{\underset{CH-COONa}{\overset{CH-COONa}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{{H}^{+}}}/{{{H}_{2}}O}\;}\underset{\text{Maleic acid}}{\mathop{\underset{CH-COOH}{\overset{CH-COOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(2) Methods of Preparation of Fumaric Acid
(i) From maleic acid :
\[\underset{\text{Maleic acid}}{\mathop{\underset{H-C-COOH}{\overset{H-C-COOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{\text{boil}}{\mathop{\xrightarrow{HCl}}}\,\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\overset{HOOC-C-H}{\mathop{\,\,\,\,\,\,\,\,\,\,|\,|}}}\,\]
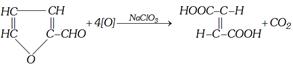
(ii) By oxidation of furfural with sodium chlorate
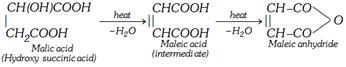
(iii) By heating malic acid at about \[150{}^\circ C\] for long time
\[\underset{\text{Malic acid}}{\mathop{\underset{C{{H}_{2}}COOH\,\,\,\,\,\,\,\,}{\overset{CH(OH)COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{150{}^\circ C,\,\,-{{H}_{2}}O}{\mathop{\xrightarrow{\text{heat}}}}\,\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\overset{HOOC-C-H}{\mathop{\,\,\,\,\,\,\,\,\,\,|\,|}}}\,\]
(iv) By heating bromosuccinic acid with alcoholic potash : By heating bromosuccinic acid with alcoholic potash.
\[\underset{CH.(Br)COOH}{\overset{C{{H}_{2}}COOH\,\,\,\,\,\,}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,\xrightarrow{\text{Alc}\text{. }KOH}\underset{\,\,\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\overset{HOOC-C-H\,\,\,\,\,\,\,\,\,}{\mathop{\,|\,|}}}\,+KBr+{{H}_{2}}O\]
(3) Physical Properties
(i) Both are colourless crystalline solids. Both are soluble in water.
(ii) The melting point of maleic acid \[(130.5{}^\circ C)\] is lower than the melting point of fumaric acid \[(287{}^\circ C)\].
(4) Chemical Properties
Chemically, both the acids give the reactions of alkenes and dibasic acids except that the maleic acid on heating forms an anhydride while fumaric acid does not give anhydride.
\[\underset{\text{Maleic acid}}{\mathop{\underset{CHCOOH}{\overset{CHCOOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{\text{hea}\text{t}}\underset{\text{Maleic anhydride}}{\mathop{\underset{CHCO}{\overset{CHCO}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,}}}\,\ \ \ \ \ O}}\,+{{H}_{2}}O\]
Both form succinic acid on reduction with sodium amalgam. They undergo addition reactions with bromine, hydrobromic acid, water, etc. and form salts, esters and acid chlorides as usual. With alkaline \[KMn{{O}_{4}}\] solution, they get oxidised to tartaric acid.
\[\underset{\begin{smallmatrix} \text{Tartaric acid} \\ \text{ (Meso)} \end{smallmatrix}}{\mathop{\underset{\underset{\,\,\,\,\,\,\,\,COOH}{\mathop{H-\underset{|}{\mathop{C}}\,-OH}}\,}{\overset{\overset{\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-OH}}\,} {\mathop{|\,\,\,}}}\,}}\,\underset{\text{(Syn-addition)}}{\mathop{\xleftarrow{\text{Alk}\text{.}KMn{{O}_{4}}}}}\,\underset{\begin{smallmatrix} \text{Maleic acid} \\ \text{ (}Cis\text{)} \end{smallmatrix}}{\mathop{\underset{H-C-COOH}{\overset{H-C-COOH}{\mathop{|\,|\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\underset{\text{(anti-addition)}}{\mathop{\xrightarrow{B{{r}_{2}}\text{water}}}}\,\underset{\text{(Racemic mixture)}}{\mathop{\underset{\underset{\,\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{Br-\underset{|}{\mathop{C}}\,-H}}\,\,\,}{\overset{\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-Br}}\,}{\mathop{|\,}}}\,}}\,\]
\[\underset{\begin{smallmatrix} \text{ Tartaric acid} \\ \text{(Racemic mixture)} \end{smallmatrix}}{\mathop{\underset{\underset{\,\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{HO-\underset{|}{\mathop{C}}\,-H\,\,\,\,\,}}\,\,\,\,\,}{\overset{\overset{\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-OH}}\,}{\mathop{|\,\,\,\,}}}\,}}\,\underset{\text{(Syn-addition)}}{\mathop{\xleftarrow{\text{Alk}\text{. }KMn{{O}_{4}}}}}\,\underset{\text{Fumaric acid (}Trans\text{)}}{\mathop{\underset{HOOC-C-H\,\,\,\,\,\,\,\,\,\,\,\,}{\overset{\,\,\,\,\,\,\,\,\,\,H-C-COOH}{\mathop{|\,|}}}\,}}\,\underset{\text{(anti-addition)}}{\mathop{\xrightarrow{B{{r}_{2}}\text{water}}}}\,\underset{\text{((Meso)}}{\mathop{\underset{\,\underset{\,\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{H-\underset{|}{\mathop{C}}\,-Br}}\,}{\overset{\overset{\,\,\,\,\,\,\,\,\,\,COOH}{\mathop{H-\overset{|}{\mathop{C}}\,-Br}}\,}{\mathop{|}}}\,}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
