Matter
Category : 5th Class
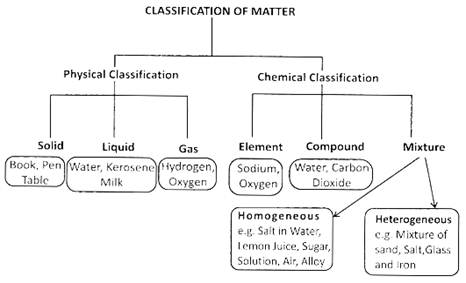
Everything, which we see around us is called the matter. Matter is made up of atoms and atoms are made up of electrons, protons, and neutrons. There are three states of matter, solid, liquid and gas. The properties of solid, liquid and gas states of a same matter are different. The atoms in the solid are tightly packed in comparison to the structure of atoms in liquid and gas. In this chapter, we will study about the properties of different types of matter.
![]() Matter
Matter
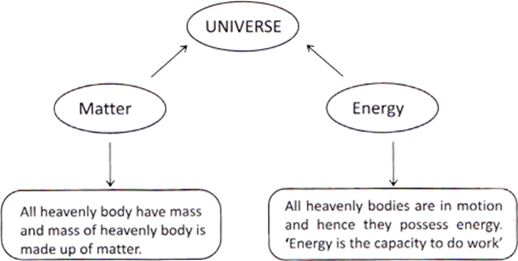
Anything which occupies space and has mass is called matter. Amount of matter present in a body is called the mass of the body. Mass of a body is constant and remains same, even in the space. Volume is the amount of space, which is occupied by the body or an object. Amount of occupied space by an object is called its volume. The whole universe is made up of only two things, matter and energy.
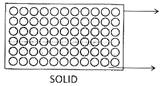
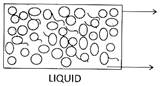
![]() Properties of Three States of Matter
Properties of Three States of Matter
| Properties | Solid | Liquid | Gas |
| Mass | It has mass | It has mass | It has mass |
| Occupy space | It occupies space | It occupies space | It occupies space |
| Shape | It has definite space | It takes the shape of container | It has no definite space |
| Intermolecular Distance | Very less, molecules tightly packed | More than solid, loosely packed molecules | Maximum, free to move in any direction |
| Intermolecular force of attrition | Maximum | Less than solid | Least |
| Fluidity | Does? t flow freely | Flows freely | Flows freely in all direction |
| Compressibility | Negligible | Compressible to some extent | Highly compressible |


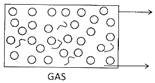
When liquid states of matter is poured Carbon dioxide, a gas produced during a
From one vessel into another, it changes chemical reaction, goes up and fills the
Its shape and acquire the shape of the empty balloon. The shows that gases
Container to which it is poured but it occupy space and always goes up
Retains its volume
![]() Molecular Arrangement in Three States of Matter
Molecular Arrangement in Three States of Matter
Force of attraction more
 Intermolecular distance is least
Intermolecular distance is least
Force of attraction less
 Intermolecular distance is more than solid
Intermolecular distance is more than solid
Force of attraction is least
 Intermolecular distance is maximum
Intermolecular distance is maximum
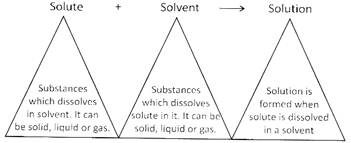
Some substances dissolve in water and some are not dissolved in water. For example, salt, sugar etc. are soluble in water whereas sand, chalk pieces are insoluble in water.
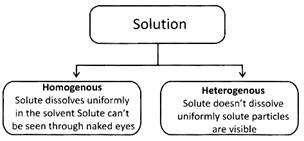
Solute, when dissolves in a liquid, occupies the empty space present between the molecules. If all of the empty space is occupied by solute and no more empty space is left between the solvent molecules, it is called saturated solution i.e. no more solute can be added to a given amount of solvent. If more solute is added, it settles at the bottom.
More solute can be added by heating the saturated solution. Solubility of a solution is increased on increasing the temperature.
![]() Element
Element
Element is the purest substance, which is made up of the same kind of atoms e.g. Hydrogen, Oxygen etc.
![]() Compound
Compound
Compound is formed when two or more than two elements are combined chemically in a fixed ratio. E.g. ![]() etc.
etc.
![]() Mixture
Mixture
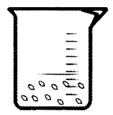
Mixture is just the physical combination of two or more than two substances and not in any fixed ratio. For example air is a mixture. Salad is also a mixture of many fruits and vegetables.
Look at the following picture of the mixture of pulse and water:
You need to login to perform this action.
You will be redirected in
3 sec
