States of Matter Solid, Liquid and Gas
Category : 4th Class
LEARNING OBJECTIVES
This lesson will help you to:—
Real Life Examples
When a container containing ice cubes is placed in sunlight it can be observed that first it melts down to form liquid water. And then if it is left undisturbed in sunlight till evening. Some of the water gets evaporated. In the atmosphere and the quantity of water in the container gets reduced.
Due to the increasing temperature of the earth, glaciers are melting, leading to the increase in the water level of the earth.
QUICK CONCEPT REVIEW
What is Matter?
STATES OF MATTER
There are three states of matter;
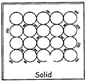
SOLID
Example: Diamond.
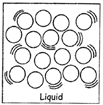
LIQUID
Example: Water.
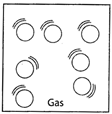
GASES
Example: Steam.
Amazing Facts
Glass is actually a liquid; it just flows very, very slowly.
Liquid oxygen is blue.
Hot water freezes quicker than cold water
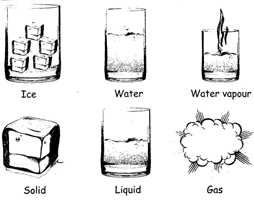
CHANGE OF STATE
Matters can be changed from one state to another state. A solid can be changed into liquid and a liquid can be changed into gas. Most of the metals, which ore solid, turn into liquid on heating and turn into vapour on further heating.
Misconcept/Concept
Misconcept: The shape of solids does not change.
Concept: Solids can be changed into another form by applying temperature or pressure.
Misconcept: There is no space between the particles of solids.
Concept: There are very small spaces between the particles of solids.
Process of change of states of matter:
SHORTCUT TO PROBLEM SOLVING
\[solid\xrightarrow{Increase\,in\,temperature}Liquid\xrightarrow{Increase\,in\,temperature}Gas\] \[Gas\xrightarrow{Decrease\,in\,temperature}Liquid\xrightarrow{Decrease\,in\,temperature}Solid\]
You need to login to perform this action.
You will be redirected in
3 sec
