Growth Hormones And Growth Regulators
Category : 11th Class
The term hormone used by first Starling (1906). He called it stimulatory substance. The growth and development in plants is controlled by a special class of chemical substances called hormones. They are needed in small quantities at very low concentrations as compared to enzyme. They are rarely effective at the site of their synthesis.
Thus, growth hormones also called phytohormones term given by Thimann (1948), it can be defined as ‘the organic substances which are synthesized in minute quantities in one part of the plant body and transported to another part where they influence specific physiological processes’. A group of plant hormones including auxins, gibberellins, cytokinins, ethylene and abscisic acid are presently known to regulate growth.
Auxins : Auxins (Gk. auxein = to grow) are weakly acidic growth hormones having an unsaturated ring structure and capable of promoting cell elongation, especially of shoots (more pronounced in decapitated shoots and shoot segments) at a concentration of less than 100 ppm which is inhibitory to the roots. Among the growth regulators, auxins were the first to be discovered.
Discovery : Julius Von Sachs was the first to indicate the presence of organ forming substances in plants. The existence of first plant growth hormone came from the work of Darwin and Darwin (1881). Darwin described the effects of light and gravity in his book, “Power of movements in plants”. Darwin and his son found that bending movement of coleoptile of Canary grass (Phalasis canariensis) was due to exposure of tip to unilateral light. Boysen-Jensen (1910; 1913) found that the tip produces a chemical which was later named auxin. Paal (1914, 1919) removed coleoptile tip and replaced it asymmetrically to find a curvature. Auxin was first collected by Went (1928) from coleoptile tip of Avena. Went also developed Avena curvature test for bioassay of auxin.
Types of auxins : There are two major categories of auxins natural auxins and synthetic auxins.
(1) Natural auxins : These are naturally occurring auxins in plants and therefore, regarded as phytohormones. Indole 3-acetic acid (IAA) is the best known and universal auxin. It is found in all plants and fungi.

The first naturally occurring auxin was isolated by Kogl and Haagen-Smit (1931) from human urine. It was identified as auxin-a (auxenotriolic acid,\[{{C}_{18}}{{H}_{32}}{{O}_{5}}\]). Later, in 1934 Kogl, Haagen-Smit and Erxleben obtained another, auxin, called auxin-b (auxenolonic acid,\[{{C}_{18}}{{H}_{30}}{{O}_{4}}\]) from corn germ oil (extracted from germinating corn seeds), and heteroauxin from human urine. Heteroauxin (C10H9O2N) also known as indole-3-acetic acid (IAA), is the best known natural auxin, Besides IAA, indole-3-acetaldehyde, indole-3-pyruvic acid, indole ethanol, 4-chloro-idole actic acid (4-chloro-IAA) etc., are some other natural auxins.
Natural auxins are synthesized (Young) in physiologically active parts of plants such as shoot apices, leaf primordia and developing seeds, buds (apex), embryos, from amino acid tryptophan. In root apices, they are synthesized in relatively very small amount. Auxins show polar movement. It is basipetal (from apex to base) in stem but acropetal (from root tip towards shoot) in the root. Auxins move slowly by diffusion from cell to cell and not through the vascular tissues. Auxins help in the elongation of both roots and shoots. However, the optimum concentration for the two is quite different.
It is 10 ppm for stem and 0.0001 ppm for the root. Higher concentration of auxins show inhibitory effect on growth.
(2) Synthetic auxins : These are synthetic compounds which cause various physiological responses common to IAA. Some of the important synthetic auxins are 2, 4-D (2, 4-dichlorophenoxy acetic acid) is the weedicide, 2, 4, 5-T (2, 4, 5-trichlorophenoxy acetic acid), IBA (indole 3-butyric acid), NAA (naphthalene acetic acid, PAA (Phenyl acetic acid), IPA (Indole 3-propionic acid). IBA is both natural and synthetic auxin. Certain compounds inhibit action of auxin and compete with auxins for active sites are called antiauxins. e.g., PCIB (p- chlorophenoxy isobutyric acid), TIBA (2, 3, 5-tri iodobenzoic acid). TIBA is used in picking cotton bolls.
Bioassay of Auxins : Testing of biological activity (growth) of a substance (auxin) by employing living material is called bioassay. Auxin bioassay is also quantitative test as it measures amount of effect in response to a particular concentration of auxin.
(1) Avena coleoptile curvature test : Avena curvature test carried out by F.W. Went (1928), demonstrated the effect of auxins on plant growth by performing some experiments with the oat (Avena sativa) coleoptile.
(2) Split pea stem curvature test : This test was also discovered by Went, 1934.
(3) Root growth inhibition test (Cress root inhibition test)
Functions of auxins : Auxins control several kinds of plant growth processes. These are as follows :
(1) Cell elongation : Auxins promote elongations and growth of stems and roots and enlargement of many fruits by stimulating elongation of cells in all directions.
The auxins cause cell enlargement by solubilisation of carbohydrates, loosening of microfibrils, synthesis of more wall materials, increased membrane permeability and respiration.
(2) Apical dominance : In many plants, the apical bud grows and the lower axillary buds are suppressed. Removal of apical bud results in the growth of lower buds. The auxin (IAA) of the terminal bud inhibits the growth of lateral buds. This phenomenon is known as apical dominance.
This property of auxins has found use in agriculture. Sprouting of lateral buds (eyes) of the potato tuber is checked by applying synthetic auxin (NAA).
(3) Control of abscission layer : Auxin inhibits abscission of leaves and fruits. Abscission layer is produced when the auxin content falls below a minimum.
Premature drop of fruits such as apple, pear and citrus can be prevented to a great extent by spraying the trees with a dilute solution of IAA, NAA or some other auxin.
(4) Weed control : Weeds are undesirable in a field with a crop. Weeds cause competition for water, mineral, light and space. This causes poor yield. By the spray of 2, 4-D, broad-leaved weeds can be destroyed but 2, 4-D does not affect mature monocotyledonous plants.
(5) Root differentiation : Many new plants are usually propogated by stem cutting e.g., Rose, Bougainvillea. If we dip the lower cut end of a cutting in dilute solution of auxins (specially IBA gives very good results) very soon large number of roots are developed on the cut ends due to which these cuttings develop into successful plants.
(6) Parthenocarpy : Parthenocarpy can be induced by application of IAA in a paste form to the stigma of a flower or by spraying the flowers with a dilute solution of IAA. Banana, oranges and grapes are now-a-days grown parthenocarpically on commercial scale.
(7) Control of lodging : In some plants when the crop is ripe and there is heavy rain accompanied by strong winds, the plants bends as a result of which the ear (inflorescence) gets submerged in water and decays. If a dilute solution of any auxin is sprayed upon young plants the possibility of bending of plants is reduced as the stem becomes stronger by the application of auxins.
(8) Flowering : In pineapple, NAA promotes flowering. In lettuce, auxins help in delaying the flowering. In cotton plants, the use of auxins increases the cotton seeds production.
(9) Differentiation of vascular tissues : Auxins induce the differentiation of xylem and phloem in intact plants and also in callus produced in vitro during tissue culture experiments.
(10) Sex expression : The spray of auxins increases the number of female flowers in cucurbits. In maize application of NAA during the period of inflorescence differentiation can induce formation of hermaphrodite or female flowers in a male inflorescence.
Thus auxins cause femaleness in plants.
(11) Healing : Healing of injury is effected through auxin induced division in the cells around the injured area. The chemical was formerly named traumatic acid or traumatin.
(12) Nodule formation : In legumes, IAA is known to stimulate nodule formation.
(13) Respiration : According to French and Beevers (1953) the auxin may increase the rate of respiration indirectly through increased supply of ADP by rapidly utilizing the ATP in the expanding cells.
Gibberellins : Gibberellins are weakly acidic hormones having gibbane ring structure which cause cell elongation of intact plants in general and increased internodal length of genetically dwarfed plants (i.e., corn, pea) in particular.
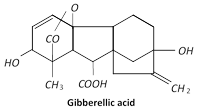
Discovery : Gibberellins were first isolated from the fungus Gibberella fujikuroi (Fusarium moniliforme) the causal organism of Bakanae disease or foolish seedling disease of rice plants in Japan by Kurosawa in 1926.
In 1939, Yabuta and Sumiki and coworkers working in Tokyo isolated an active substance from the fungus and called it Gibberellin A. This gibberellin preparation was probably a mixture of several gibberellins. The first gibberellin to be obtained was Gibberellin A-3. Cross et al. (1961) explained the detailed structure of gibberellic acid. Now 60 gibberellins have been identified from different groups of plants.
Many of them occur naturally in plants. Gibberella fujikuroi has as many as 15 gibberellins. All the different types of gibberellins, known so far, have gibbane skeleton and are acidic in nature. Therefore, these are termed as\[G{{A}_{1}}({{C}_{19}}{{H}_{24}}{{O}_{6}}),G{{A}_{2}}({{C}_{19}}{{H}_{26}}{{O}_{6}}),G{{A}_{3}}({{C}_{19}}{{H}_{22}}{{O}_{6}}),G{{A}_{4}}({{C}_{19}}{{H}_{24}}{{O}_{5}})\]and so on. Of these gibberellic acid or gibberellin \[{{A}_{3}}(G{{A}_{3}})\] is the commonest. Gibberellins are synthesised in plants in leaves of buds, developing embryos, root tips, young apical leaves, shoot tips and seeds. Gibberellins are transported readily in the plant, apparently moving passively in the stream either in xylem or phloem. Their transport in non-polar. Anti-gibberellins like malic hydrazide, phosphon D, Alar and chorocholine cheoride (CCC) or cycocel are also called antiretardants (stimulates flowering and inhibits the growth of nodes). Commercial production of GA is still carried out by culturing this fungus in large vats.
Bioassay of gibberellin : Gibberellin bioassay is performed through dwarf maize/pea test and cereal endosperm test.
(1) Dwarf pea bioassay : Seeds of dwarf pea are allowed to germinate till the just emergence of plumule. GA solution is applied to some seedlings others are kept as control. After 5 days, epicotyl length is measured. Increase in length of epicotyl over control seedlings is proportional to GA concentration.
(2) Barley endosperm bioassay : Endosperms are detached from embryos, sterilized and allow to remain in 1ml of test solution for \[1-2\] days. There is build up of reducing sugars which is proportional to GA concentrations. Reducing sugars do not occur in endosperms kept as control.
Functions of gibberellin
(1) Stem elongation : The gibberellins induce elongation of the internodes. The elongation of stem results due to rapid cell division and cell elongation induced by gibberellins.
(2) Leaf expansion : In many plants leaves become broader and elongated when treated with gibberellic acid. This leads to increase in photosynthetic area which finally increases the height of the plant. Interestingly, gibberellins show no effect on roots.
(3) Reversal of dwarfism : One of the most striking effects of gibberellins is the elongation of genetic dwarf (mutant) varieties of plants like corn and pea. It is believed that dwarfism in the mutant variety of plant is due to blocking of the capacity for normal gibberellin production (deficiency of gibberellin). When gibberellin is applied to single gene dwarf mutants e.g., Pisum sativam, Vicia faba and Phaseolus multiflorus, they grow to their nomal heights. It is further interesting to note that application of gibberellins to normal plants fail to show any remarkable effects.
(4) Bolting and Flowering : Gibberellins induce stem elongation in ‘rosette plants’ e.g., cabbage, henbane, etc. Such plants show retarded internodal growth and profuse leaf development. In these plants just prior to the reproductive phase, the internodes elongate enormously causing a marked increase in stem height. This is called bolting.
Bolting needs long days or cold nights. It has been further noticed that if cabbage head is kept under warm nights, it remains vegetative. The exogenous application of gibberellins induced bolting in first year itself in plants like cabbage (normally bolting occurs next year due to effect of endogenous gibberellins).
(5) Enzyme formation : One of the most dramatic effects of GA is its induction of hydrolytic enzymes in the aleurone layer of endosperm of germinating barley seeds and cereal grains. GA stimulates the production of digestive enzymes like proteases, a-amylases, lipases which help to mobilise stored nutrients. GA treatment stimulates a substantial synthesis of new mRNA. Thus GA acts to uncover or depress specific genes, which then cause the synthesis of these enzymes. It is assumed that GA acts on the DNA of the nucleus.
(6) Breaking of dormancy : Gibberellins overcome the natural dormancy of buds, tubers, seeds, etc. and allow then to grow. In this function gibberellins act antagonistically to abscisic acid (ABA).
(7) Parthenocarpy : Gibberellins have been considered to be more effective than auxins for inducing parthenocarpy in fruits like apple, tomato and pear. GA application has also resulted in the production of large fruits and bunch length in seedless grapes.
(8) Sex expression : Gibberellins control sex expression in certain plants. In general, gibberellin promote the formation of male flowers either in place of female flowers in monoecious plants such as cucurbits or in genetically female plants like Cannabis, Cucumis.
(9) Substitution for vernalization : Vernalization is the low temperature requirement of certain plant (i.e., biennials) to induce flowering. The low temperature requirement of biennials for flowering can be replaced by gibberellins.
(10) Malt yield : There is increased malt production when gibberellins are provided to germinating barley grains (due to greater production of a-amylase).
(11) Delayed ripening : Ripening of citrus fruits can be delayed with the help of gibberellins. It is useful in safe and prolonged storage of fruits.
(12) Seed germination : Gibberellins induce germination of positively photo-blastic seeds of lettuce and tobacco in complete darkness.
Cytokinins (Phytokinins) : Cytokinins are plant growth hormones which are basic in nature, either aminopurine or phenyl urea derivatives that promote cell division (cytokinesis) either alone or in conjugation with auxin.
Discovery : The first cytokinin was discovered by Miller, Skoog and Strong (1955) during callus tissue culture of Nicotiana tobaccum (tobacco).
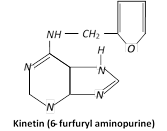
It was synthetic product formed by autoclaving Herring sperm (fish sperm) DNA. This synthetic product was identified as 6-furfuryl amino-purine and named as kinetin. He found that normal cell division induced by adding yeast extract.
Various terms such as kinetenoid (Burstran, 1961), phytokinin (Dendolph et al. 1963) phytocytomine (Pilet 1965) have been used for kinetin like substances but the term cytokinin proposed by Letham (1963) has been widely accepted. Letham et al. (1964) discovered first natural, cytokinin in unripe maize grain (Zea mays). It was named as zeatin (6 hydroxy 3 methyl trans 2-butenyl amino purine).
About 18 cytokinins have been discovered, e.g., dihydrozeatin, IPA (Isopentenyl adenine), benzyl adenine. The most widely occurring cytokinin in plant is IPA. It has been isolated from Pseudomonas tumefaciens. Many are found as constituents of tRNAs. Cytokinins are synthesized in roots as well as endosperm of seeds. Coconut millk and Apple fruit extract are rich in cytokinins. Cytokinins in coconut milk called coconut milk factor.
Kinetin (6 furfuryl amino purine) is a derivative of the nitrogen base adenine. Plant physiologists use the term cytokinins to designate group of substances that stimulate cell division in plants. Cytokinins are produced in actively growing tissues such as embryos, developing fruits and roots. Kinetin is the derivative of purine base adenine, which bears furfuryl group at 9 position which migrated to 6 position of the adenine ring during autoclaving of DNA.
Cytokinin is transported to different parts of the plant through xylem elements. According to Osborne and Black (1964), the movement of cytokinin is polar and basipetal.
Bioassay of cytokinins : Bioassay is done through retention of chlorophyll by leaf discs, gains of weight of a tissue in culture, excised radish cotyledon expansion etc.,
(1) Tobacco pith culture : Tobacco pith culture is divided into two weighted lots one supplied with cytokinin and the other without it. After 3-5 weeks, increase of fresh weight of treated tissue over control is noted. It is a measure of stimulation of cell division and hence cytokinin activity.
(2) Retardation of leaf senescence : Leaves are cut into equal sized discs with the help of a cutter. They are devided into two lots. One lot is provided with cytokinin. After 48-72 hours, leaf discs are compared for chlorophyll contents. Cytokinin retards chlorophyll degradation.
(3) Excised radish cotyledon expansion : Excised radish cotyledons are measured and placed in test solution as well as ordinary water (as control). Enlargement of cotyledons indicates cytokinin activity.
(4) Root inhibition test : Kiraly and his coworkers (1966) used root inhibition test for cytokinin bioassay. They found, that amount of root inhibition of actively growing seedlings is related to cytokinin activity.
Functions of cytokinins
(1) Cell division : Cytokinins are essential for cytokinesis and thus promote cell division. In presence of auxin, cytokinins stimulate cell division even in non-meristematic tissues. In tissue cultures, cell division of callus (undifferentiated mass of parenchyma tissue) is enhanced when both auxin and cytokinin are present. But no response occurs with auxin or cytokinin alone.
(2) Cell enlargement and Differentiation : Under some conditions cytokinins enhance the expansion of leaf cells in leaf discs and cotyledons. These cells considered to be mature and under normal conditions do not expand. Cytokinins play a vital role in morphogenesis and differentiation in plants. It is now known that kinetin-auxin interaction control the morphogenetic differentiation of shoot and root meristems.
(3) Delay in senescence : Cytokinin delay the senescence (ageing) of leaves and other organs by controlling protein synthesis and mobilization of resources (Disappearance of chlorophyll). It is called Richmond Lang effect. It was reported by Richmond and Lang (1957) while working on detached leaves of Xanthium.
(4) Counteraction of apical dominance : Auxins and cytokinins act antagonistically in the control of apical dominance. Auxins are responsible for stimulating growth of apical bud. On the other hand, cytokinins promote the growth of lateral buds. Thus exogenous application of cytokinin has been found to counteract the usual dominance of apical buds.
(5) Breaking of dormancy : Cytokinins breaks seeds dormancy of various types and thus help in their germination. They also induce germination of positively photoplastic seed like lettuce and tobacco even in darkness.
(6) Accumulation and Translocation of solutes : Cytokinins induce accumulation of salts inside the cells. They also help solute translocation in phloem.
(7) Sex expression : Cytokinins promote formation of female flowers in some plants.
(8) Enzyme activity : Cytokinins stimulate the activity of enzymes especially those concerned with photosynthesis.
(9) Parthenocarpy : Development of parthenocarpic fruits through cytokinin treatment has been reported by Crane (1965).
(10) Pomalin : A combination of cytokinin (6-benzladenine) and gibberellin \[(G{{A}_{4}},G{{A}_{7}})\] called pomalin is particularly effective in increasing apple size.
(11) Initiation of interfasicular cambium : Cytokinins induce the formation of interfasicular cambium in plants e.g., Pinus radiata.
(12) Nucleic acid metabolism : Guttman (1957) found a quick increase in the amount of RNA in the nuclei of onion root after kinetin treatment.
(13) Protein synthesis : Osborne (1962) demonstrated the increased rate of protein synthesis on kinetin treatment.
(14) Flowering : Gibberellins also play an important role in the initiation of flowering. Lang (1960) demonstrated that added gibberellin could substitute for the proper environmental conditions in Hyoscyamus niger which requires long day treatment for flowering. Such effects of gibberellin are common among vernalised and long day plants.
Gibberellin is also known to play essential role in germination of cereal seeds.
Ethylene : Ethylene is a gaseous hormone which stimulates transverse growth but retards the longitudinal one.
Discovery : The effect of ethylene had been known since long. Kerosene lamps and hay have been used by fruit merchants to hasten colour development (ripening) in fruits. These effects are due to ethylene. Neljubow (1901) observed that ethylene gas alters the tropic responses of roots. Denny (1924) reported that ethylene induces ripening of fruits. Crocker et al. (1935) identified ethylene as gaseous plant hormone.
Ethylene is produced in plants from the amino acid methionine. It is synthesized in almost all plant parts-roots, leaves, flowers, fruits, seeds. It is more synthesized in nodal regions. Maximum synthesis of ethylene occurs during climacteric ripening of fruits. High concentration of auxin induce ethylene formation. When a fruit ripens its respiration rate gradually decreases, but when it is reversed by a sharp increase called climactric. Some of the inhibitory effects earlier attributed to auxin are known to be caused by ethylene.
The commercial product for providing ethylene is ethaphon (2-chloroethyl phosphoric acid). Ethaphon is a liquid from which ethylene gas is released, hence this substance is used for artificial ripening of fruits.
Bioassay of ethylene : It is done on the principle of triple response which includes three characteristic effects of ehtylene on etiolated seedlings of pea-viz.
(1) Triple pea test : Pratt and Biale (1944) developed this method for bioassay of ethylene which base on the physiological effect of ethylene to cause
(2) Pea stem swelling test : Cherry (1973) used pea seedlings to measure ethylene concentration by marked increase of stem swelling expressed as a ratio of weight to length. In one ppm of ethylene the ratio is about 4.0.
Functions of ethylene
(1) Fruit growth and Ripening : Ethylene promotes fruit growth and its ripening. The hormone is used in the artificial ripening of climacteric fruits (e.g., Apple, Banana, Mango).
(2) Transverse growth : Ethylene inhibits longitudinal growth but stimulates transverse growth so that stem looks swollen.
(3) Epinasty (leaf bending) : Epinasty represents more growth on upper surface of leaf than on lower surface. Epinasty is said to be controlled by ethylene in many plants.
(4) Abscission : Ethylene stimulates formation of abscission zone in leaves, flowers and fruits.
(5) Apical dominance : Ethylene inhibits the growth of lateral buds and thus cause apical dominance (in pea). It is believed that auxin might be functioning partly through synthesis of ethylene in causing apical dominance.
(6) Root initiation : In low concentration, ethylene stimulates root initiation and growth of lateral roots and root hair.
(7) Flowering : Ethylene stimulates flowering in pineapple and related plants though in other cases, the hormone causes fading of flowers. Fading flowers of Vanda are known to release ethylene. Sleep disease (inrolling of petals in blossomed flowers) in due to ethylene.
(8) Sex expression : Ethylene application increases the number of female flowers and fruits in cucumber plants.
(9) Dormancy : It breaks dormancy of different plant organs but not of lateral buds.
Abscisic acid (ABA) : Abscisic acid is a mildly acidic growth hormone, which functions as a general growth inhibitor by counteracting other hormones (auxin, gibberellins, cytokinins) or reactions mediated by them.
Discovery : The hormone was first isolated by Addicott et al. (1963) from cotton balls. They named it as abscisin II. Simultaneously, Wareing and Cornforth isolated a substance that can induce bud dormancy. They named the substance as dormin. Later, both these substances were found to be the same and were named as abscisic acid. It is produced in many parts of the plants but more abundantly inside the chloroplasts of green cells. The synthesis of abscisic acid is stimulated by drought, water logging and other adverse environmental conditions. Therefore, it is also called stress hormone. The hormone is formed from mevalonic acid or xanthophylls. Chemically it is dextro-rotatory cis sesquiterpene. The hormone is transported to all parts of the plant through diffusion as well as through conductive channels.
In some plant tissues (especially in young shoots) occurs a related compound called xanthoxine.
Whether xanthoxine is an intermediate of the ABA-biosynthesis or whether it is an independent product remains unknown. The structure indicates that both ABA and xanthoxine are terpene derivatives.
Bioassay of abscisic acid
(1) Rice seedling growth inhibition test : Mohanty, Anjaneyulu and Sridhar (1979) used rice growth inhibition method to measure ABA like activity. The length of second leaf sheath after six days of growth is measured.
(2) Inhibition of a-amylase synthesis in barley endosperm test : ABA inhibits the synthesis of a-amylase in the aleurone layers which is triggered by gibberellins. Goldschmidt and Monselise (1968) developed the bioassay method to estimate ABA activity by determining the extent of inhibition of a-amylase synthesis induced by treating barley seed endosperm with GA.
Functions of abscisic acid
(1) Control : It keeps growth under check by counter acting the effect of growth promoting hormones, i.e., auxins, cytokinins and gibberellins. As growth is primarily controlled by gibberellins, abscisic acid is popularly called antigibberellic hormone. It will inhibit seed germination, growth of excised embryos, growth of Duckweed and other plants.
(2) Dormancy : Abscisic acid acts as growth inhibitor and induces dormancy of buds towards the approach of winter. Dormancy of seeds is mainly caused by abscisic acid. Because of its action in inducing dormancy abscisic acid (ABA) is also called dormin. The buds as well as seeds sprout only when abscisic acid is overcome by gibberellins.
(3) Abscission : ABA promotes the abscission of leaves, flowers and fruits in plants.
(4) Senescence : Abscisic acid stimulates senescence of leaves by causing destruction of chlorophyll (an effect opposite to that of cytokinins) and inhibition of protein and RNA synthesis. The effect, however, can be reversed by application of cytokinins in Lemna.
(5) Antitranspirant : Abscisic acid can be used as antitranspirant. Application of minute quantity of ABA to leaves reduces transpiration to a great extent through partial closure of stomata. It thus conserves water and reduces the requirement of irrigation.
(6) Hardiness : Abscisic acid promotes cold hardiness and inhibits growth of pathogens.
(7) Flowering : ABA delays flowering in long day plants. However, in some short day plants (e.g., strawberry, black current) it promotes flowering.
(8) Rooting : Abscisic acid can be used to promote rooting in many stem cuttings.
Wound hormone or Traumatic acid or Necrohormone : Haberlandt (1913) reported that injured plants cells release a chemical substance (wound hormone), which stimulate the adjacent cells to divide rapidly in order to heal up the wound. English et al. (1939) finally isolated and crystallized this wound hormone and named it as Traumatic acid. Although traumatic acid has been found to be very active in inducing meristematic activity in uninjured green bean pods, but it is not effective in most of the plant tissues including tobacco pith tissues.
Morphactins : Morphactins are synthetic growth regulators which act in variety of ways on the natural regulation mechanisms of plants. The important ones are phenoxyalkancarboxylic acid (synthetic auxin), substituted benzoic acids, Malic acid hydrazide, Fluorene-9 carboxylic acids and their derivatives, Chlorflurenol, Chloroflurun, Flurenol, Methylbenzilate, Dichlorflurenol, etc. Morphactins have fundamental action on morphogenesis of plants and this characteristic designation (morphactins) is derived from morphologically active substances.
The actions of these substances are systematic and after their uptake they are transported and distributed not polarly (as seen by IAA) but basi- and acropetally. Generally these are growth inhibitors. These contain ‘fluorene ring’ in their structure.
Functions
(1) Seed germination : In general, morphactins inhibit germination seeds particularly the emergence of the radicle from the seed shell. This property can be counteracted with GA3 and almost completely by cytokinins. The germination of fern spores is also delayed by morephactins.
(2) Growth seedling : Morphactins inhibit the growth of seedling affecting the shoot and often also root. With this property they show a similarity with cytokinin. The inhibitory effect of seedling shoot growth can be partly counteracted with GA3 but not the inhibition of root growth.
(3) Stem elongation : They have inhibitory effect on the stem elongation. Increased concentration produces dwarfing in the plants.
(4) Polarity of cell division : Denffer and others (1969) observed in the dividing cells of the root tips of Allium that treatment of morphactin (CFI) results in random orientation of the mitotic spindle and plane of cell division, i.e., they exercise depolarisation during cell division.
Jasmonic acid (Jasmonates) : According to Parthier (1991), jasmonic acid and its methyl esters are ubiquitous in plants. They have hormone properties, help regulating plant growth, development and they seem to participate in leaf senescence and in the defense mechanism against fungi.
Just like ABA jasmonates inhibit a premature germination of the oil-containing seeds of Brassica and Linum. After germination they induce the synthesis of the seed storage proteins Napin and Cruciferin as well as that of several more elaiosome-associated proteins.
Calines (Formative hormones) : Certain other natural growth hormones in plants called as calines or formative hormone which are through to be essential for the effect of auxin an root, stem and leaf growth they are :
(1) Rhizocaline or Root forming hormone : It is produced by the leaves and translocated in a polar manner down the stem.
(2) Caulocaline or Stem forming hormone : It is produced by the roots and is transported upward in the stem.
(3) Phyllocaline or Self forming hormone : It is produced probably by the cotyledons. It stimulates mesophyll development in the leaves and is synthesized only in the presence of light.
You need to login to perform this action.
You will be redirected in
3 sec
