Modern Periodic Classification
Category : 10th Class
![]() Periodic Law
Periodic Law
'Properties of elements are a periodic function of their atomic number Atomic number gives us the number of protons in the nucleus of an atom and this number increases by one in going from one element to the next. Elements, when arranged in order of increasing atomic number Z, lead us to the classification known as the Modern Periodic Table. Prediction of properties of elements could be made with more precision when elements were arranged on the basis of increasing atomic number.
![]() Position of Elements in the Modern Periodic Table:
Position of Elements in the Modern Periodic Table:
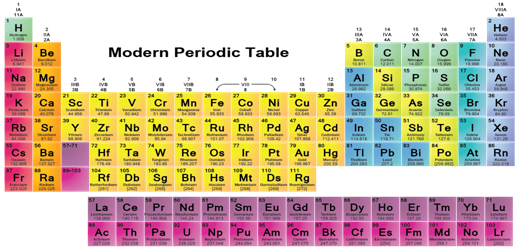
The Modern Periodic Table has 18 vertical columns known as 'groups' and 7 horizontal rows known as 'periods'. Let us see what decides the placing of an element in a certain group and period. All elements of a group contain same number of valence electrons, which justifies similar chemical properties. The atomic radius decreases in moving from left to right along a period. This is due to an increase in nuclear charge, which tends to pull the electrons closer to the nucleus and reduces the size of the atom. Atoms of different elements with the same number of occupied shells are placed in the same period. Na,
Mg, Al, Si, P, S, Cl and Ar belong to the third period of the Modern Periodic
Table, since the electrons in the atoms of these elements are filled in K, L and M shells.
![]() Metallic & Non-metallic Properties
Metallic & Non-metallic Properties
Metals like Na and Mg are towards the left-hand side of the Periodic Table while the non-metals like sulphur and chlorine are found on the right-hand side. In the middle, we have silicon, which is classified as a semi-metal or metalloid because it exhibits some properties of both metals and non-metals. In the Modern Periodic Table, a zig-zag line separates metals from non-metals. The borderline elements - boron, silicon, germanium, arsenic, antimony, tellurium and polonium - are intermediate in properties and are called metalloids or semi-metals. Metals tend to lose electrons while forming bonds, that is, they are electropositive in nature.
As the effective nuclear charge acting on the valence shell electrons increases across a period, the tendency to lose electrons decreases. Down the group, the effective nuclear charge experienced by valence electrons is decreasing because the outermost electrons are farther away from the nucleus. Therefore, these can be lost easily. Hence metallic character decreases across a period
and increases down a group.
As the trends in the electro negativity show, non-metals are found on the right- hand side of the Periodic Table towards the top. These trends also help us to predict the nature of oxides formed by the elements.
It is know that the oxides of metals are basic and that of non-metals are acidic in general.
![]() Groups
Groups
A group or family is a vertical column in the periodic table. Groups are considered the most important method of classifying the elements. In some groups/the elements have very similar properties and exhibit a clear trend in properties down the group. Under the international naming system, the groups are numbered numerically 1 through 18 from the left most column (the alkali metals) to the right most column (the noble gases). The older naming systems differed slightly between Europe and America . These groups tend to be given trivial (unsystematic) names, e.g., the alkali metals, alkaline earth metals, halogens, pnictogens, chalcogens, and noble gases. Some other groups in the periodic table display fewer similarities and vertical trends (for example group 7). These have no trivial names and are referred to simply by their group numbers.
Elements in the same group show patterns in atomic radius, ionization energy, and electronegativity. From top to bottom in a group, the atomic radii of the elements increase. Since there are more filled energy levels, valence electrons are found farther from the nucleus. From the top, each successive element has a lower ionization energy, as it is easier to remove an electron since the atoms are less tightly bound. Similarly, a group has a top to bottom decrease in electronegativity due to an increasing distance between valence electrons and the nucleus.
![]() Periods
Periods
A period is a horizontal row in the periodic table. Although groups are the most common way of classifying elements, there are some regions of the periodic table where the horizontal trends and similarities in properties are more significant than vertical group trends. This can be true in the d-block (or "transition metals"), and especially for the f-block, where the lanthanides and actinides form two substantial horizontal series of elements. Periodic trend for ionization energy. Each period begins at a minimum for the alkali metals, and ends at a maximum for the noble gases. Elements in the same period show trends in atomic radius, ionization energy, electron affinity, and electronegativity. Moving left to right across a period, atomic radius usually decreases. This occurs because each successive element has an added proton and electron which causes the electron to be drawn closer to the nucleus. This decrease in atomic radius also causes the ionization energy to increase when moving from left to right across a period. The more tightly bound an element is, the more energy is required to remove an electron. Electronegativity increases in the same manner as ionization energy, because of the pull exerted on the electrons by the nucleus. Electron affinity also shows a slight trend across a period. Metals (left side of a period) generally have a lower electron affinity than nonmetals (right side of a period) with the exception of the noble gases.
![]() Removal of Anomalies in Mendeleev's Periodic Table
Removal of Anomalies in Mendeleev's Periodic Table
Thus the modern periodic table of elements removed all anomalies of the Mendeleev's periodic table, by simple considering the atomic numbers of elements.
![]() The Features of the Periodic Table are
The Features of the Periodic Table are
(i) The 1st period is the shortest period. It consists of just two elements H and He.
(ii) The 2nd and the 3rd periods have 8 elements each and are called short periods.
(iii) The 4th and the 5th periods are long periods and have 18 elements each.
(iv) The 6th period has 32 elements. The period has a 15 element series called
Lanthanide series, separated from the table. The lanthanide series are rare earth elements that show similar properties.
(v) The 7th period contains all the rest of the elements. It is incomplete. This period also has a 15 element series called the Actinide series, separated from the table. The actinide series have a separate identity and contains uranium and most of the known transuranic elements.
(i) The groups are divided into A and B groups.
(ii) Group 1A to VIII A has all the normal elements.
(iii) Group 1B to VIII B holds all the transition metal elements.
(iv) The other two groups are the lanthanide and the actinide series. They are also known as inner transition elements.
![]()
The first period starts with hydrogen (H) and ends with helium (He). It has just two elements H (Z = 1) and He (Z = 2). H has one electron in the K-shell. He has 2 electrons in the K-shell. As we have seen in the chapter on the structure of atoms, the K-shell can hold only 2 electrons. Thus the first period is complete. It has to be borne in mind that the place of hydrogen is unique in the periodic table. It has been placed above the alkali elements starting with Li in group 1A. This is because H has valency 1 just as the other alkali elements. But the properties of hydrogen otherwise are very different from the other group 1A alkali elements Li, Na, K, Cs, etc.
Now let us see the next periods : periods 2 and 3. The second period starts with Li (Z=3), where the K-shell is filled and the next shell, the L-shell is starting to fill. After Li the next element is beryllium (Be, Z=4). Its K-shell is complete and it has 2 electrons in the L-shell. The maximum number of electrons held in the L-shell is 8. So the period has 8 elements, in which each element's L-shell is getting filled. The last element in the period is neon (Ne, Z=10). Neon's both K and the L shell are completely filled. A similarly situation occurs for the third period. Here the next shell after L-shell, namely the M-shell is getting filled. The maximum number of electrons in the M-shell is 8. Thus across the period, starting with element sodium (Na, Z=11) the M-shell has 1 electron; and the period ends with argon (Ar, Z= 18) which has 2 electrons in the K-shell, 8. electrons in the L-shell and 8 electrons in the M-shell. In the fourth period, the N-shell starts to fill. N shell can hold a maximum of 2 electrons. Hence K has 1 electron in the N-shell and the next element Ca contains 2 electrons in the N-shell. Now the N-shell is complete. After Ca, there are 10 elements starting from Scandium (Sc) to Zinc (Zn). Here the next shell of electrons is filling. This shell can contain a maximum of 10 electrons. The next shell can have a maximum of 6 electrons. Hence the fourth period is long and has 18 elements. The same is true for the fifth period. In the sixth and the seventh period, the electrons start to fill a new orbital (f- shell). This has been separated out as the Lanthanide and the Actinide series. Now let us look at some of the chemical and physical properties in a particular period. What we will. learn from one period, will hold true for all the other periods. Consider the third period. The figure below shows how the electronic configuration is changing as we go from left to right in the period. The number of valence electrons is increasing in an integral fashion. The change in the valency is according to the tendency to give up or borrow electrons. Thus elements in the same period have consecutive atomic numbers and different valencies. If we see the atomic radii across the period, we will notice that the size decreases across the period. Now let us consider the metallic character of the elements in the third period.
We have proper metals in the first and the second places : sodium (Na) and magnesium (Mg) are alkali and alkaline-earth metals. They give up the electrons in the last shell very easily. They are shiny in colour and conduct electricity. After Mg comes aluminum (Al). Al has 3 electrons in its outermost shell and behaves like a metal. The next element is silicon (Si). It has 4 electrons in its outermost shell. It thus needs to borrow four electrons or give up all its four electrons to form a stable shell. Si does not do any of these, instead it binds tetrahedrally most of the time. Thus Si behaves neither like a metal nor like a non-metal. Hence it is called-as a metalloid. After Si, come three elements : phosphorus (P), sulphur (S) and chlorine (Cl). All the three are non-metals. Thus, while moving from left to right in the period, the metallicity decreases. Also the chemical reactivity first decreases and then increases. As discussed before, the chemical reactivity depends on how easily the outermost orbit gives off or borrows electrons to make a stable orbit. The two extremes of the third period, namely Na and Cl are very reactive. But Na is very electropositive in nature, where as Cl is very electro-negative in nature.

![]() Who gave modern periodic law?
Who gave modern periodic law?
(a) Newland
(b) Mendeleev's
(c) Moseley
(d) Luther Mayer
(e) None of these
Answer: (c)
![]() What was the basis of classification of modern periodic law?
What was the basis of classification of modern periodic law?
(a) Atomic Number
(b) Atomic Mass
(c) Valency
(d) Isotopes
(e) None of these
Answer: (a)
![]() Which group elements are known as alkali metals?
Which group elements are known as alkali metals?
(a) 1
(b) 2
(c) 3
(d) 4
(e) None of these
Answer: (a)
![]() Who gave octate periodic law?
Who gave octate periodic law?
(a) Newland
(b) Mendeleev's
(c) Moseley
(d) Luther Mayer
(e) None of these
Answer: (a)
![]() Which one of the following is most reactive?
Which one of the following is most reactive?
(a) F
(b) Cl
(c) Br
(d) I
(e) None of these
Answer: (a)

![]()
You need to login to perform this action.
You will be redirected in
3 sec
