Metallurgy
Category : 10th Class
In order to extract a pure metal from its ores various steps are to be followed which depends upon the nature of ore and reactivity of metal. In general the following steps are followed for extraction of metals from their ores: Dressing of ore (crushing/grinding) \[\to \] Isolation of metal from concentrated \[\to \] orerefining of extracted metal \[\to \] Pure metal Thus, metallurgy is defined as the process involved in the extraction of metals from their ores.
![]() Dressing of Ore
Dressing of Ore
The ore is first crushed into small pieces and then ground to fine powder in stamp mills,
![]() Concentration or Enrichment of Ore
Concentration or Enrichment of Ore
The ore extracted from earth contains impurities like sand, stone, saw dust etc. Depending upon the nature of ore and the impurities present any one of the following methods can be used.
![]() Hydraulic Washing or Gravity Separation Method
Hydraulic Washing or Gravity Separation Method
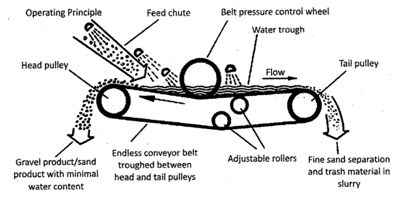
This method is used when the ore particles are heavier than the impurities. The powdered ore is placed over a wooden inclined table. It is then sprayed with a stream of water which carries away the lighter impurities with it. The heavier ores are left behind on the wooden table.
![]() Magnetic Separation
Magnetic Separation
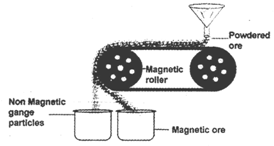
This method is used when either ore or the impurity is magnetic. It is carried on a conveyor belt moving on two rollers one of which is magnetic. As the ores falls down from the magnetic roller, the magnetic particles of the ore or impurities and the non-magnetic particles take up two different positions and are collected in two heaps.
![]() Froth Flotation Method
Froth Flotation Method
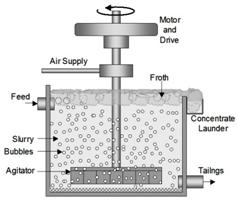
This method is used for the concentration of sulphide ores. The powdered ore is mixed and agitated strongly and froth is produced. The sulphide ore particles are preferentially waited by the forth and are carried to the surface while the impurities settles at the bottom. The froth is removed and washed and then we get the concentrated ore.
![]() Chemical Separation
Chemical Separation
This method is used when the ore contain large impurities and their effective separation is not possible by physical methods.

For concentrating bauxite ore the powered ore is mixed with hot and concentrated solution of sodium hydroxide. The ore forms soluble sodium metal aluminate, while the impurities remains insoluble which is filtered and removed. The filtrate is then hydrolyzed to get aluminium hydroxide precipitate. This precipitate is filtered and dried and then heated strongly to get alumina.
\[A{{l}_{2}}{{O}_{3}}+2NaOH\to 2NaAl{{O}_{2}}+{{H}_{2}}O\]
\[Si{{O}_{2}}+2NaOH\to N{{a}_{2}}Si{{O}_{3}}+{{H}_{2}}O\]
\[NaAl{{O}_{2}}+2{{H}_{2}}O\to Al\,{{(OH)}_{3}}+NaOH\]
\[2Al\,{{(OH)}_{3}}\to A{{l}_{2}}{{O}_{3}}+3{{H}_{2}}O\]
![]() Conversion of Enriched Ore into the Metal Oxide
Conversion of Enriched Ore into the Metal Oxide
![]() Calcinations
Calcinations
It involves heating of the ore below its fusion temperature in absence of air. This step expels organic matter and moisture from the ores. It can remove moisture from hydrated oxides or carbon dioxide from carbonates.
e.g. \[A{{l}_{2}}{{O}_{3}}.2{{H}_{2}}O\to A{{l}_{2}}{{O}_{3}}+2{{H}_{2}}O\]
\[2F{{e}_{2}}{{O}_{3}}.3{{H}_{2}}O\to 2F{{e}_{2}}{{O}_{3}}+6{{H}_{2}}O\]
\[CuC{{O}_{3}}.Cu{{(OH)}_{2}}\to 2CuO+C{{O}_{2}}+{{H}_{2}}O\]
Calcination makes the ore porous. The step is generally performed in reverberatory furnace.
![]() Roasting
Roasting
It is the heating of the ore either alone or with some other material usually in presence of air below its fusion temperature. Roasting is generally done in a reverberatory furnace or in a blast furnace. In roasting definite chemical changes like oxidation, chlorination, etc., take place. While in calcination there occurs only expulsion of organic matter, water, carbon dioxide etc., i.e., it does not involve any major chemical change. The roasting process may be any one of the following types :
![]() Oxidizing Roasting
Oxidizing Roasting
This is very common type of roasting in metallurgy and is carried out to remove sulphur and arsenic in the form of their volatile oxides such as \[S{{O}_{2}}\] and \[A{{S}_{2}}{{O}_{3}}\] respectively. The ores are simultaneously converted into corresponding oxides. This type of roasting is generally applied in ores of lead, zinc, nickel, copper, etc.
\[S+{{O}_{2}}\to S{{O}_{2}}\]
\[4As+3{{O}_{2}}\to 2A{{s}_{2}}{{O}_{3}}\]
\[2PbS+3{{O}_{2}}\to 2PbO+2S{{O}_{2}}\]
\[2CuS+3{{O}_{2}}\to 2C{{u}_{2}}O+\,2S{{O}_{2}}\]
![]() Partial Oxidizing of Sulphating Roasting
Partial Oxidizing of Sulphating Roasting
This type of roasting is carried out at a temperature below the melting point of the charge and air is admitted. Part of the sulphide ore is oxidized to sulphate and the rest is converted into oxide. For example, the roasting of galena leads to the formation of a mixture of lead oxide and lead sulphate.
\[2PbS+3{{O}_{2}}\to 2PbO+2S{{O}_{2}}\]
\[PbS+S{{O}_{2}}\to PbS{{O}_{4}}\]
![]() Chlorinating Roasting
Chlorinating Roasting
This type of roasting is done in the case of silver ore. The ore, argentite is mixed with common salt and the mixture is heated in the presence of air. The final product is the chloride of the metal.
\[A{{g}_{2}}S+2NaCl\to 2AgCl+N{{a}_{2}}S\]
![]() Leaching
Leaching
It involves the treatment of the ore with a suitable reagent as to make it soluble, while impurities remain insoluble. The ore is recovered from the solution by suitable chemical method. For example, bauxite ore contains ferric oxide, titanium oxide and silica as impurities. When the powdered ore is digested with an aqueous solution of sodium hydroxide at about 150°C under pressure, the alumina \[(A{{l}_{2}}{{O}_{3}})\] dissolves forming soluble sodium metaaluminate while ferric oxide \[(Fe2{{O}_{3}}),\,Ti{{O}_{2}}\] and silica remain as insoluble part.
\[A{{l}_{2}}{{O}_{3}}+2NaOH\to 2NaAl{{O}_{2}}+{{H}_{2}}O\]
Pure alumina is recovered from the filtrate
\[NaAl{{O}_{2}}+2{{H}_{2}}O\to Al{{(OH)}_{3}}+NaOH\]
\[2Al{{(OH)}_{3}}\to A{{l}_{2}}{{O}_{3}}+3{{H}_{2}}O\]
Gold and silver are also extracted from their native ores by Leaching (Mac-Arthur Forrest cyanide process). Both silver and gold particles dissolves in dilute solution of sodium cyanide in presence of oxygen of the air forming complex cyanides.
\[4Ag+8NaCN+2{{H}_{2}}O+{{O}_{2}}\to 4NaAg{{(CN)}_{2}}+4NaOH\]
Sod. Argento cyanide
\[4Au+8NaCN+2{{H}_{2}}O+{{O}_{2}}\to 4NaAu{{(CN)}_{2}}+4NaOH\]
\[Ag\] or \[Au\] is recovered from the solution by the addition of electropositive metal like zinc.
\[2NaAg{{(CN)}_{2}}+Zn\to N{{a}_{2}}Zn{{(CN)}_{4}}+2Ag\]
\[2NaAu{{(CN)}_{2}}+Zn\to N{{a}_{2}}Zn{{(CN)}_{4}}+2Au\]
![]() Reduction to Free Metal
Reduction to Free Metal
Some of the methods commonly used to get free metal from the concentrated ore are given below :
![]()
This involves the reduction of the ore to the molten metal at a high temperature For the extraction of less electropositive metals such as \[Pb,\,\,Zn,\,\,Fe,\,\,Sn,\,\] etc., powerful reducing agents such as \[C,\,\,{{H}_{2}},\,\,CO,\] water gas, \[Na,\,\,K,\,\,Mg,\,\,Al\] may be used.
Some examples are given below :
\[PbO+C\to Pb+CO\]
\[CuO+CO\to Cu+C{{O}_{2}}\]
\[TiC{{l}_{4}}+2Mg\to Ti+2MgC{{l}_{2}}\]
\[C{{r}_{2}}{{O}_{3}}+2Al\to 2Cr+A{{l}_{2}}{{O}_{3}}\]
Out of these carbon reduction and aluminium reduction are important processes.
![]() Carbon Reduction Process
Carbon Reduction Process
It is generally called as smelting. The oxides of the less electropositive metals are reduced by strongly heating them with coal or coke.
\[PbO+C\to Pb+CO\]
\[PbO+CO\to Pb+C{{O}_{2}}\]
Similarly,
\[F{{e}_{2}}{{O}_{3}}+3C\to 2Fe+3CO\]
\[F{{e}_{2}}{{O}_{3}}+3CO\to 2Fe+3C{{O}_{2}}\]
The ores, even after concentration, contain some gangue. To remove gangue, certain substances are mixed with concentrated ore which combine with gangue to form fusible material which is not soluble in molten metal. The substances used are called fluxes and the fusible material formed during reduction process is called slag. Slag is usually lighter and floats on the surface of the molten metal. An acidic flux (e.g., silica, borax, etc.) is the chemical substance which removes the basic impurities.
\[Si{{O}_{2}}+CaO\to CaSi{{O}_{3}}\]
The basic flux (e.g., limestone, magnesite, ferric oxide, etc.) is the chemical substance which removes the acidic impurities.
\[MgC{{O}_{3}}+Si{{O}_{3}}\to MgSi{{O}_{3}}+C{{O}_{2}}\]
![]() Reduction by Alumnium
Reduction by Alumnium
This process is employed in the case of those metals which have very high melting points and are to be extracted from their oxides. Their reduction with carbon is not satisfactory. A mixture of concentrated oxide ore and aluminium powder, commonly called as thermite, is taken in a steel crucible placed in a bed of sand. The reaction is started by the use of an ignition mixture containing magnesium powder and barium peroxide.
\[C{{r}_{2}}{{O}_{3}}+2Al\to 2Cr+A{{l}_{2}}{{O}_{3}}\]
\[3M{{n}_{3}}{{O}_{4}}+8Al\to 9Mn+4A{{l}_{2}}{{O}_{3}}\]
Large amount of heat energy is released during reduction, which fuses both the alumina and the metal.
![]() Self Reduction Process
Self Reduction Process
This process is also called autoreduction process or air reduction process. The sulphide ores of less electropositive metals like \[Hg,\,\,Pb,\,\,Cu,\] etc., are heated in air as to convert part of the ore into oxide or sulphate which then reacts with the remaining sulphide ore to give the metal and sulphur dioxide. No external reducing agent is used in this process.
\[2HgS+3{{O}_{2}}\to 2HgO+2S{{O}_{2}}\] Extraction of \[Hg\]
\[2HgO+HgS\to 3Hg+S{{O}_{2}}\] from cinnabar ore
\[2PbS+3{{O}_{2}}\to 2PbO+2S{{O}_{2}}\], Extraction of lead
\[2PbO+PbS\to 3Pb+S{{O}_{2}}\], from galena ore
![]() Electrolytic Reduction
Electrolytic Reduction
The oxides of the highly electropositive metals like Na, K, Mg, Ca, Al, etc., cannot be reduced easily with carbon at moderate temperatures. For reduction, a very high temperature is required at which the metal may combine with carbon to form a carbide. These metals are thus extracted by the electrolysis of their oxides, hydroxides or chlorides in fused state. Sometimes, a small amount of some other salt is added as to lower the fusion temperature or to increase the conductivity or both. The metal is liberated at the cathode. Sodium is obtained by the electrolysis of fused mixture of \[NaCl\] and \[CaC{{l}_{2}}\], (Down's process) or by electrolysis of fused sodium hydroxide (Castner's process).
![]() Hydrometallurgy
Hydrometallurgy
This process is based on the fact that more electropositive metal can displace less electropositive metal from its salt solution. The ore is teated with such chemical reagents which convert it into soluble compound. By the addition of more electropositive metal to the filtrate, the metal present in the ore can be precipitated. The following two examples illustrate this process.
(i) Extraction of Copper
Malachite ore is first roasted.
\[CuC{{O}_{3}}.Cu{{(OH)}_{2}}\to 2CuO+{{H}_{2}}O+C{{O}_{2}}\]
Copper oxide obtained is dissolved in sulphuric acid.
\[CuO+{{H}_{2}}S{{O}_{4}}\to CuS{{O}_{4}}+{{H}_{2}}O\]
To the solution of copper sulphate, scrap iron is added which precipitates copper.
\[CuS{{O}_{4}}+Fe\to Cu+FeS{{O}_{4}}\]
(ii) Extraction of Silver
The ore is dissolved in sodium cyanide solution.
\[A{{g}_{2}}S+4NaCN\to 2NaAg{{(CN)}_{2}}+N{{a}_{2}}S\]
By the addition of zinc turnings, Ag is precipitated
\[2NaAg{{(CN)}_{2}}+Zn\to N{{a}_{2}}Zn{{(CN)}_{4}}+2Ag\]
A which combines with the particles of the metal present in the ore and form amalgam. The metal is recovered from the amalgam by subjecting it to distillation where the mercury distills over leaving behind the metal.
![]() Third Operation-Refining
Third Operation-Refining
The metals obtained by the application of above reduction methods from the concentrated ores are usually impure. These impure metals may be associated with small amounts of (a) unchanged ore, (b) other metals produced by the simultaneous reduction of their compounds originally present in the ore, (c) non-metals like silicon, carbon, phosphorus, etc. (d) residual slag, flux, etc. The impure metal is thus subjected to some purifying processes known as refining, in order to remove the undesired impurities. The following refining processes, may be applied depending upon the nature of the metal under treatment and the nature of the impurities.
![]() Liquation Process
Liquation Process
This process is based on the difference in fusibility of the metal and impurities. When the impurities are less fusible than the metal itself, this process is employed. The metal melts and flows down leaving behind the impurities on the hearth. This method is used to purify the metals like \[Bi,\,\,Sn,\,\,Pb,\,\,Hg,\] etc.
![]() Distillation
Distillation
This process is used for those metals which are easily volatile. The impure metal is heated in a retort and its vapours are separately condensed in a receiver. The non-volatile impurities are left-behind in the retort. This is used for the purification of \[Zn,\,\,Cd,\,Hg,\] etc.
![]() Electrolytic Refining of Metals
Electrolytic Refining of Metals
Many of the metals such as copper, silver, gold, aluminium, lead, etc., are purified by this method. This is perhaps the most important method. The impure metal is made anode, while a thin sheet of pure metal acts as a cathode. The electrolytic solution consists of generally an aqueous solution of a salt or a complex of the metal. On passing the current, the pure metal is deposited on the cathode and equivalent amount of the metal gets dissolved from the anode. Thus, the metal is transferred from anode to cathode through solution. The soluble impurities pass into the solution while the insoluble one, especially less electropositive impurities collect below the anode as anodic mud or anode sludge,. Some examples are given below :
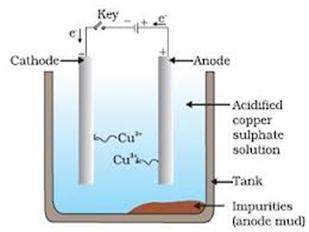
(i) Purification of copper
Impure metal—Anode; Thin sheets of copper—Cathode
Electrolyte—An aqueous solution of copper sulphate containing \[{{H}_{2}}S{{O}_{4}}\].
A current of 1.3 volt is used. Anodic mud contains \[Ag,\,Au,\,\,Pt,\,\,Pd,\] etc., and impurities like Fe, Zn, Mi, etc., pass into the solution. 99.9% pure copper is obtained,
![]() Corrosion
Corrosion
When the metals are left exposed to air and moisture, its free surface starts decomposing. This decomposition of free surface of metallic surface when left exposed to the air and moisture is called corrosion. When the corrosion takes place on iron then it is called rusting. Rusting occurs only in presence of air and moistures. It is an electrochemical process in which different parts of the iron surface acts as electrodes in a cell reaction. At the anode, iron atoms dissolve as \[F{{e}^{2+}}\] ions.
\[Fe\to F{{e}^{2+}}+2e\]
At cathode, hydroxide ions are formed.
\[{{O}_{2}}+{{H}_{2}}O+4e\to 4O{{H}^{-}}\]
\[4Fe+3{{O}_{2}}+{{H}_{2}}O\to Fe2{{O}_{3}}+2Fe{{(OH)}_{3}}\]
The rusts is presumably \[F{{e}_{2}}{{O}_{3}}\] and \[Fe{{(OH)}_{3}}\]. It increases in presence of impurities and acid in water.
![]() Prevention of corrosion
Prevention of corrosion
By alloying: We can prevent corrosion by making alloys with non reactive metals.

![]()
You need to login to perform this action.
You will be redirected in
3 sec
