Hydrocarbons
Category : 10th Class
A hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls. Aromatic hydrocarbons (arenes), alkanes, alkenes, cycloalkanes and alkyne-based compounds are different types of hydrocarbons.
The majority of hydrocarbons found naturally occur in crude oil, where decomposed organic matter provides an abundance of carbon and hydrogen which, when bonded, can catenate to form millions of chains.
![]() Types of Hydrocarbons
Types of Hydrocarbons
The classifications for hydrocarbons defined as follows:
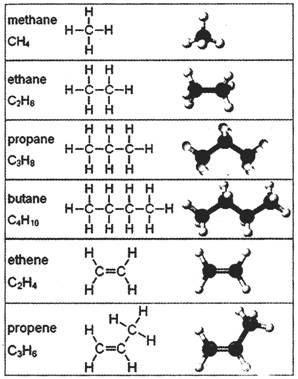
Saturated hydrocarbons (alkanes) are the simplest of the hydrocarbon species and are composed entirely of single bonds and are saturated with hydrogen. The general formula for saturated hydrocarbons is\[{{\mathbf{C}}_{n}}{{\mathbf{H}}_{2n+2}}\]. Saturated hydrocarbons are the basis of petroleum fuels and are either found as linear or branched species. Hydrocarbons with the same molecular formula buts’ different structural formulae are called structural isomers. Methylhexane and its higher homologues, branched hydrocarbons can be chiral. Unsaturated hydrocarbons have one or more double or triple bonds between carbon atoms. Those with double bond are called alkenes. Those with one double bond have the formula \[{{\mathbf{C}}_{n}}{{\mathbf{H}}_{2n}}\]. Those containing triple bonds are called alkynes, with general formula \[{{\mathbf{C}}_{n}}{{\mathbf{H}}_{2n-2}}\].
Cycloalkanes are hydrocarbons; containing one or more carbon rings to which hydrogen atoms are attached. The general formula fora saturated hydrocarbon containing one ring is \[{{\mathbf{C}}_{n}}{{\mathbf{H}}_{2n}}\].
| Molecular | Name | Number of Carbon atoms | Prefix | Melting Point (C) | Boiling Point | Physical State |
| Methane | 1 | Meth | -183 | -162 | Gas | |
| Ethan | 2 | Eth | -172 | -89 | Gas | |
| Propane | 3 | Prop | -187 | -42 | Gas | |
| Butane | 4 | But | -138 | 0 | Gas | |
| Pentane | 5 | Pent | -130 | 36 | Liquid | |
| Hexane | 6 | Hex | -95 | 69 | Liquid | |
| Heptane | 7 | Hept | -91 | 98 | Liquid | |
| Octane | 8 | Oct | -57 | 126 | Liquid | |
| Nonane | 9 | Non | -54 | 151 | Liquid | |
| Decane | 10 | Dec | -30 | 174 | Liquid |
Aromatic hydrocarbons, also known as arenes, are hydrocarbons that have at least one aromatic ring.
Hydrocarbons can be gases (e.g. methane and propane), liquids (e.g. hexane and benzene), waxes or low melting solids (e.g. paraffin wax and naphthalene) or polymers (e.g. polyethylene, polypropylene and polystyrene).
![]() General Properties
General Properties
Because of differences in molecular structure, the empirical formula remains different between hydrocarbons; in linear, or straight-run alkanes, alkenes and alkynes.
The amount of bonded hydrogen lessens in alkenes and alkynes due to the self-bonding or catenation of carbon preventing entire saturation of the hydrocarbon by the formation of double or triple bonds.
![]() Hydrocarbons and Formula
Hydrocarbons and Formula
| Sl. No. | Alkanes | Molecular Formula |
Alkenes | Molecular Formula |
Alkynes | Molecular Formula |
| 1. | Methan | |
*** | *** | *** | *** |
| 2. | Ethane | Ethene | Ethne | |||
| 3. | Propane | Propene | Propyne | |||
| 4. | Butane | Butane | Butyne | |||
| 5. | Pentane | Pentene | Pentyne | |||
| 6. | Hexane | Hexane | Hexyn |
![]() Usage
Usage
Hydrocarbons are one of the Earth's most important sources of energy. The important use of hydrocarbons is as a combustible fuel. In their solid form, hydrocarbons take the form of asphalt. Mixtures of volatile hydrocarbons are now used in preference to the chlorofluorocarbons as a propellant for aerosol sprays, due to chlorofluorocarbon's impact on the ozone layer. Methane and ethane are gaseous at ambient temperatures and cannot be readily liquefied by pressure alone. Propane is however easily liquefied, and exists in 'propane bottles' mostly as a liquid. Butane is so easily liquefied that it provides a safe, volatile fuel for small pocket lighters. Pentane is a clear liquid at room temperature, commonly used in chemistry and industry as a powerful nearly odorless solvent of waxes and high molecular weight organic compounds, including greases. Hexane is also a widely used non-polar, non- aromatic solvent, as well as a significant fraction of common gasoline.
![]() Burning Hydrocarbons
Burning Hydrocarbons
Hydrocarbons are currently the main source of the world's electric energy and heat sources, because of the energy produced when burnt. Often this energy is used directly as heat, such as, in home heaters, which use either oil or natural gas. The hydrocarbon is burnt and the heat is used to heat water, which is then circulated. A similar principle is used to create electric energy in power plants.
Common properties of hydrocarbons are the facts that they produce steam, carbon dioxide and heat during combustion and that oxygen is required for combustion to take place. The simplest hydrocarbon, methane, burns as follows:
\[C{{H}_{4}}+2{{O}_{2}}\to 2{{H}_{2}}O+C{{O}_{2}}+\]Energy
Another example of this property is propane:
\[{{C}_{3}}{{H}_{8}}+5{{O}_{2}}\to 4{{H}_{2}}O+3C{{O}_{2}}+\]Energy
\[{{C}_{n}}{{H}_{2n+2}}+\left( \frac{3n+1}{2} \right){{O}_{2}}\to (n+1)\,{{H}_{2}}O+n\,C{{O}_{2}}+\]Energy
Burning of hydrocarbons is an example of exothermic chemical reaction.
![]() Petroleum
Petroleum
Oil refineries are key to obtaining hydrocarbons. Crude oil is processed in several stages to form desired hydrocarbons, used as fuel and in other products. Extracted hydrocarbons in a liquid form are referred to as petroleum, whereas hydrocarbons in a gaseous form are referred to as natural gas. Petroleum and natural gas are found in the Earth's subsurface with the tools of petroleum geology and are a significant source of fuel and raw materials for the production of organic chemicals.
The extraction of liquid hydrocarbon fuel from sedimentary basins is integral to modern energy development. Hydrocarbons are mined from tar sands and oil shale, and potentially extracted from sedimentary methane hydrates.
Hydrocarbons are economically important.
Because major fossil fuels such as coal, petroleum and natural gas, and its derivatives such as plastics, paraffin, waxes, solvents and oils are hydrocarbons. Hydrocarbons along with \[N{{O}_{2}}\] and sunlight contribute to the formation of tropospheric ozone and greenhouse gases.

![]() Which one of the following is not a hydrocarbon?
Which one of the following is not a hydrocarbon?
(a) \[C{{H}_{4}}\]
(b) \[{{C}_{2}}{{H}_{5}}OH\]
(c) \[C{{H}_{3}}COOH\]
(d) \[NaOH\]
(e) None of these
Answer: (d)
![]() Which one of the following is a structure of alkenes?
Which one of the following is a structure of alkenes?
(a) 
(b) 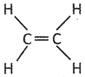
(c) ![]()
(d) All of these
(e) None of these
Answer: (b)
![]() The general formula for alkyne is:
The general formula for alkyne is:
(a) \[{{C}_{n}}{{H}_{2n+1}}\]
(b) \[{{C}_{n}}{{H}_{2n}}\]
(c) \[{{C}_{n}}{{H}_{2n-1}}\]
(d) \[{{C}_{n}}{{H}_{2n+2}}\]
(e) None of these
Answer: (c)
![]() The boiling point of methane is
The boiling point of methane is
(a) -162 °C
(b) -82 °C
(c) -64 °C
(d) -120 °C
(e) None of these
Answer: (a)
![]() Which one of the following is liquid at room temperature?
Which one of the following is liquid at room temperature?
(a) Methane
(b) Ethane
(c) Butane
(d) Heptane
(e) None of these
Answer: (d)
You need to login to perform this action.
You will be redirected in
3 sec
