Category : NEET
Heat of Reaction
Heat of reaction is defined as the amount of heat evolved or absorbed when quantities of the substances indicated by the chemical equation have completely reacted. The heat of reaction (or enthalpy of reaction) is actually the difference between the enthalpies of the products and the reactants when the quantities of the reactants indicated by the chemical equation have completely reacted. Mathematically,
Enthalpy of reaction (heat of reaction) \[=\Delta H=\Sigma {{H}_{P}}-\Sigma {{H}_{R}}\]
(1) Factors which influence the heat of reaction : There are a number of factors which affect the magnitude of heat of reaction.
(i) Physical state of reactants and products : Heat energy is involved for changing the physical state of a chemical substance. For example in the conversion of water into steam, heat is absorbed and heat is evolved when steam is condensed. Considering the following two reactions
\[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)={{H}_{2}}O(g);\] \[\Delta H=-\,57.8\,kcal\]
\[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)={{H}_{2}}O(l);\] \[\Delta H=-\,68.32\,kcal\]
It is observed that there is difference in the value of \[\Delta H\] if water is obtained in gaseous or liquid state. \[\Delta H\] value in second case is higher because heat is evolved when steam condenses. Hence, physical sate always affects the heat of reaction.
(ii) Allotropic forms of the element : Heat energy is also involved when one allotropic form of an element is converted into another. Thus, the value of \[\Delta H\] depends on the allotropic form used in the reaction. For example, the value of \[\Delta H\] is different when carbon in the form of diamond or in amorphous form is used.
C (diamond) \[+{{O}_{2}}(g)\,\to C{{O}_{2}}(g);\] \[\Delta H=-\,94.3\,kcal\]
C (amorphous) \[+{{O}_{2}}(g)\to C{{O}_{2}}(g);\] \[\Delta H=-\,97.6\,kcal\]
The difference between the two values is equal to the heat absorbed when 12g of diamond is converted into 12g of amorphous carbon. This is termed as heat of transition.
(iii) Temperature : Heat of reaction has been found to depend upon the temperature at which reaction is occurring. The variation of the heat of reaction with temperature can be ascertained by using Kirchhoff's equation.
\[\frac{\Delta {{H}_{{{T}_{2}}}}-\Delta {{H}_{{{T}_{1}}}}}{{{T}_{2}}-{{T}_{1}}}=\Delta {{C}_{P}}\]
Kirchhoff's equation at constant volume may be given as,
\[\frac{\Delta {{E}_{{{T}_{2}}}}-\Delta {{E}_{{{T}_{1}}}}}{{{T}_{2}}-{{T}_{1}}}=\Delta {{C}_{\nu }}\]
(iv) Reaction carried out at constant pressure or constant volume : When a chemical reaction occurs at constant volume, the heat change is called the internal energy of reaction at constant volume. However, most of the reactions are carried out at constant pressure; the enthalpy change is then termed as the enthalpy of reaction at constant pressure. The difference in the values is negligible when solids and liquids are involved in a chemical change. But, in reactions which involve gases, the difference in two values is considerable.
\[\Delta E+\Delta nRT=\Delta H\] or \[{{q}_{v}}+\Delta nRT={{q}_{p}}\]
\[\Delta E\] =\[{{q}_{v}}=\] heat change at constant volume; \[\Delta H\]=\[{{q}_{p}}=\] heat change at constant pressure,
\[\Delta n=\text{total number of moles of gaseous product - total number of moles of gaseous reactants}\text{.}\]
(2) Types of heat of reaction
(i) Heat of formation : It is the quantity of heat evolved or absorbed (i.e. the change in enthalpy) when one mole of the substance is formed from its constituent elements under given conditions of temperature and pressure. It is represented by \[\Delta {{H}_{f}}\]. When the temperature and pressure are as \[{{25}^{\text{o}}}C\] and 1 atmospheric pressure. The heat of formation under these conditions is called standard heat of formation. It is usually represented by \[\Delta H_{f}^{0}\].
The standard heat of formation of 1 mole of \[N{{H}_{3}}(g)\] and \[1\] mole of \[HCl\,(g)\].
\[\frac{1}{2}{{N}_{2}}(g)+\frac{3}{2}{{H}_{2}}(g)\to N{{H}_{3}}(g);\] \[\Delta {{H}_{(g)}}=-11kcal\]
\[\frac{1}{2}\]\[{{H}_{2}}(g)+\frac{1}{2}C{{l}_{2}}(g)\to HCl\]; \[\Delta {{H}_{f}}=-22\,kcal\]
It may be calculated by
\[\Delta {{H}^{0}}=\left[ \begin{matrix}
\text{Sum of standard heats of } \\
\text{Formation of products} \\
\end{matrix} \right]\,-\,\left[ \begin{matrix}
\text{Sum of standard heats of} \\
\text{ Formation of reactants} \\
\end{matrix} \right]\]
\[\Delta {{H}^{0}}=[\Sigma \Delta {{H}^{0}}_{(\text{products)}}\text{ }\Sigma \Delta {{H}^{0}}_{(\text{Reactants)}}]\]
(ii) Heat of combustion : It is the amount of heat evolved or absorbed (i.e. change in enthalpy) when one mole of the substance is completely burnt in air or oxygen. For example
\[C{{H}_{4}}(g)\ +\ 2{{O}_{2}}(g)\ \to \ C{{O}_{2}}(g)\ +\ 2{{H}_{2}}O(l);\ \ \ \,\,\,\,\,\,\ \Delta H\ =\ -\ 192\ kcal\]
\[{{C}_{2}}{{H}_{6}}(g)\ +\ 3.5\ {{O}_{2}}(g)\ \to \ 2C{{O}_{2}}(g)\ +\ 3{{H}_{2}}O(l);\ \ \Delta H\ =\ -\ 372.8\ kcal\]
It may be calculated by
\[\Delta {{H}^{0}}=\left[ \begin{matrix}
\text{Sum of the standard heats of } \\
\text{Combustion of products} \\
\end{matrix} \right]\,-\,\left[ \begin{matrix}
\text{Sum of the standard heats } \\
\text{ Combustion of reactants} \\
\end{matrix} \right]\]
\[\Delta {{H}^{0}}=[\Sigma \Delta H{{_{f}^{0}}_{(\text{Products)}}}\,\text{-}\,\Sigma H{{_{f}^{0}}_{(\text{Reactants)}}}\text{ }\!\!]\!\!\text{ }\]
Note : Heat of combustion increases with increase in number of carbon and hydrogen.
Heat of combustion of carbon is equal to the intrinsic energy of \[C{{O}_{2}}\].
The enthalpy or heat of combustion have a number of applications. Some of these are described below,
(a) Calorific value of foods and fuels : Energy is needed for the working of all machines. Even human body is no exception. Coal, petroleum, natural gas etc. serve as the principal sources of energy for man-made machines, the food which we eat serves as a source of energy to our body.
The energy released by the combustion of foods or fuels is usually compared in terms of their combustion energies per gram. It is known as calorific value. The amount of heat produced in calories or Joules when one gram of a substance (food or fuel) is completely burnt or oxidised.
When methane burns, 890.3 kJ mol?1 of energy is released.
\[\underset{1\text{ mole (16g)}}{\mathop{C{{H}_{4}}(g)+}}\,2{{O}_{2}}(g)\to C{{O}_{2}}(g)+2{{H}_{2}}O(l);\] \[\Delta {{H}_{C{{H}_{4}}}}=-890.3\,kJ\]
So, the calorific value of methane = \[-\frac{890.3}{16}=-55.6kJ/g\]
Calorific values of some important food stuffs and fuels
|
Fuel |
Calorific value (kJ/g) |
Food |
Calorific value (kJ/g) |
|
Wood |
17 |
Milk |
3.1 |
|
Charcoal |
33 |
Egg |
6.7 |
|
Kerosene |
48 |
Rice |
16.7 |
|
Methane |
55 |
Sugar |
17.3 |
|
L.P.G. |
55 |
Butter |
30.4 |
|
Hydrogen |
150 |
Ghee |
37.6 |
Out of the fuels listed, hydrogen has the highest calorific value. The calorific value of proteins is quite low.
(b) Enthalpies of formation : Enthalpies of formation of various compounds, which are not directly obtained, can be calculated from the data of enthalpies of combustions easily by the application of Hess's law.
(iii) Heat of neutralization: It is the amount of heat evolved (i.e., change in enthalpy) when one equivalent of an acid is neutralised by one equivalent of a base in fairly dilute solution, e.g., Neutralisation reactions are always exothermic reaction and the value of \[\Delta H\] is \[(-ve)\].
\[HCl(aq.)\ +\ NaOH\ (aq.)\ \to \ NaCl(aq.)\ +\ {{H}_{2}}O;\ \ \ \ \Delta H\ =\ -\ 13.7\ kcal\]
The heat of neutralisation of a strong acid against a strong base is always constant \[(13.7\ kcal\]or \[57\ kJ\ mol{{e}^{-1}})\]. It is because in dilute solutions all strong acids and bases ionise completely and thus the heat of neutralisation in such cases is actually the heat of formation of water from \[{{H}^{+}}\] and \[O{{H}^{-}}\] ions, i.e.,
\[{{H}^{+}}\ +\ O{{H}^{-}}\ \to \ {{H}_{2}}O;\Delta H\ =\ -\ 13.7\ kcal\]
In case of neutralisation of a weak acid or a weak base against a strong base or acid respectively, since a part of the evolved heat is used up in ionising the weak acid or base, it is always less than \[13.7\ kcal\ mol{{e}^{-1}}\ (57\ kJ\ mol{{e}^{-1}})\]. For example, heat of neutralisation of \[HCN\] (a weak acid) and \[NaOH\] (a strong alkali) is \[-2.9\ kcal\] because \[10.8\ kcal\] of heat is absorbed for the ionisation of \[HCN\] (i.e., the heat of dissociation or ionisation of \[HCN\] is \[10.8\ kcal\]) Similarly Heat of neutralization of \[N{{H}_{4}}OH\] and \[HCl\] is less then 13.7 kcal.
\[HCN\ (aq.)\ +\ NaOH\ (aq.)\ \to \ NaCN(aq.)\ +\ {{H}_{2}}O;\ \ \ \Delta H\ =\ -\ 2.9\ kcal\]
\[HCN\ (aq.)\ \]⇌ H+ + CN?; DH = 10.8 Kcal
(iv) Heat of solution : It is the amount of heat evolved or absorbed (i.e., change in enthalpy) when one mole of the solute is dissolved completely in excess of the solvent (usually water). For example,
\[N{{H}_{4}}Cl(s)\ +\ {{H}_{2}}O(l)\ \to \ N{{H}_{4}}Cl(aq.);\ \ \ \ \ \Delta H\ =\ +\ 3.90\ kcal\]
\[BaC{{l}_{2}}(s)\ +\ {{H}_{2}}O(l)\ \to \ BaC{{l}_{2}}(aq.);\ \ \ \ \ \ \ \ \Delta H\ =\ -\ 2.70\ kcal\]
(v) Heat of hydration : It is the amount of heat evolved or absorbed (i.e change in enthalpy) when \[1\]mole of an anhydrous or a partially hydrated salt combines with the required number of moles of water to form a specific hydrate. For example, \[CuS{{O}_{4}}(s)\ +\ 5{{H}_{2}}O(l)\ \to \ CuS{{O}_{4}}.\ 5{{H}_{2}}O(s);\ \ \Delta H\ =\ -\ 18.69\]
(vi) Heat of vapourisation : When a liquid is allowed to evaporate, it absorbs heat from the surroundings and evaporation is accompanied by increase in enthalpy. For example: \[10.5\ k\]cals is the increase in enthalpy when one mole of water is allowed to evaporate at \[{{25}^{o}}C\]. When the vapours are allowed to condense to liquid state, the heat is evolved and condensation of vapour is accompanied by decrease in enthalpy. The value for the condensation of one mole of water vapour at \[{{25}^{\text{o}}}C\] is also \[10.5\ k\]cals.
The evaporation and condensation can be represented as,
\[{{H}_{2}}O(l)\ \to \ {{H}_{2}}O(g);\ \ \Delta H\ =\ +\ 10.5\ kcals\ \ (+43.93\ kJ)\]
\[{{H}_{2}}O(g)\ \to \ {{H}_{2}}O(l);\ \ \ \Delta H\ =\ -\ 10.5\ kcals\ \ \ (-43.93\ kJ)\]
Thus the change in enthalpy when a liquid changes into vapour state or when vapour changes into liquid state is called heat of vapourisation.
(vii) Heat of fusion : When a solid is allowed to melt, it changes into liquid state with the absorption of heat (increase in enthalpy) and when a liquid is allowed to freeze, it changes into solid with the evolution of heat (decrease in enthalpy). The change in enthalpy of such type of transformations is called enthalpy of fusion. For example,
\[{{H}_{2}}O(ice)\ \to \ {{H}_{2}}O(liquid);\ \ \ \ \Delta H\ =\ +\ 1.44\ kcals\ \ (+\,6.02\ kJ)\]
\[{{H}_{2}}O\ (liquid)\ \to \ {{H}_{2}}O\ (ice);\ \ \ \ \Delta H\ =\ -\ 1.44\ kcals\ (-\ 6.02\ kJ)\]
Note: The enthalpy of fusion of ice per mole is 6 kJ.
(viii) Heat of precipitation : It is defined as the amount of heat liberated in the precipitation of one mole of a sparingly soluble substance when solutions of suitable electrolytes are mixed, for example
\[B{{a}^{2+}}+SO_{4}^{2-}(aq)\to BaS{{O}_{4}}(s)\,:\,\,\,\Delta H=-\,4.66\,kcal\]
(ix) Heat of sublimation : Sublimation is a process in which a solid on heating changes directly into gaseous state below its melting point.
Heat of sublimation of a substance is the amount of heat absorbed in the conversion of 1 mole of a solid directly into vapour phase at a given temperature below its melting point.
\[{{I}_{2}}(s)\to {{I}_{2}}(g)\]; \[\Delta H=+\,62.39\,kJ\]
Most solids that sublime are molecular in nature e.g. iodine and naphthalene etc.
\[\Delta {{H}_{sub.}}=\Delta {{H}_{\text{fusion}}}+\Delta {{H}_{vaporisation}}\]
(3) Experimental determination of the heat of reaction : The heat evolved or absorbed in a chemical reaction is measured by carrying out the reaction in an apparatus called calorimeter. The principle of measurement is that heat given out is equal to heat taken, i.e., \[Q=(W+m)\times s\times ({{T}_{2}}-{{T}_{1}}),\]
Where Q is the heat of the reaction (given out), W is the water equivalent of the calorimeter and m is the mass of liquid in the calorimeter and s its specific heat, T2 is the final temperature and \[{{T}_{1}}\] the initial temperature of the system. Different types of calorimeters are used but two of the common types are,
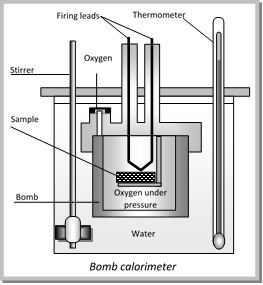
(i) Water calorimeter and (ii) Bomb calorimeter
Bomb calorimeter : This is commonly used to find the heat of combustion of organic substances. It consists of a sealed combustion chamber called a bomb. A weigh quantity of the substance in a dish along with oxygen under about 20 atmospheric pressure is placed in the bomb which is lowered in water contained in an insulated copper vessel. The vessel is fitted with a stirrer and a sensitive thermometer. The arrangement is shown in fig.
The temperature of the water is noted and the substance is ignited by an electric current. After combustion the rise in temperature of the system is noted. The heat of combustion can be calculated from the heat gained by water and calorimeter.
Since the reaction in a bomb calorimeter proceeds at constant volume, the heat of combustion measured is \[\Delta E\]
\[\Delta E=\frac{(W+m)\,({{t}_{2}}-{{t}_{1}})\times s}{{{w}_{1}}}\times M\,kcal\]
Where M is the molecular mass of the substance, \[{{w}_{1}}\] is the weight of substance taken, W is the water equivalent of calorimeter, m is the mass of liquid in the calorimeter and s is the specific heat of liquid.
\[\Delta H\] can be calculated from the relation, \[\Delta H=\Delta E+\Delta nRT\]
You need to login to perform this action.
You will be redirected in
3 sec
