Category : Exam Preparation Tips
In chemical kinetics, the order of reaction with respect to a given substance (such as reactant, catalyst or product) is defined as the index, or exponent, to which its concentration term in the rate equation is raised. For the typical rate equation of form r; =; k[mathrm{A}]^x[mathrm{B}]^y..., where [A], [B], ... are concentrations, the reaction orders (or partial reaction orders) are x for substance A, y for substance B, etc. The overall reaction order is the sum x + y + ....
For example, the chemical reaction between mercury (II) chloride and oxalate ion
2 HgCl2(aq) + C2O42-(aq) → 2 Cl-(aq) + 2 CO2(g) + Hg2Cl2(s)
has the observed rate equation
r = k[HgCl2]1[C2O42-]2
In this case, the reaction order with respect to the reactant HgCl2 is 1 and with respect to oxalate ion is 2; the overall reaction order is 1 + 2 = 3. As is true for many reactions, the reaction orders (here 1 and 2 respectively) differ from the stoichiometric coefficients (2 and 1). Reaction orders can be determined only by experiment. Their knowledge allows conclusions to be drawn about the reaction mechanism, and may help to identify the rate-determining step.Elementary (single-step) reactions do have reaction orders equal to the stoichiometric coefficients for each reactant, but complex (multi-step) reactions may or may not have reaction orders equal to their stoichiometric coefficients.Orders of reaction for each reactant are often positive integers, but they may also be zero, fractional, or negative.A reaction can also have an undefined reaction order with respect to a reactant if the rate is not simply proportional to some power of the concentration of that reactant; for example, one cannot talk about reaction order in the rate equation for a bimolecular reaction between adsorbed molecules:
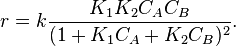
Visit our site http://www.studyadda.com/videos/xii-class-chemistry-lectures/chemical-kinetics/order-of-reaction/1758
All the video lectures at StudyAdda are exclusively developed and taught by Mr. Lalit Sardana(IIT-JEE,AIR 243) and Mrs. Shweta Sardana(AIPMT Trainer, MSc., MPhil (Gold Medalist)), Sardana Tutorials.
You need to login to perform this action.
You will be redirected in
3 sec
